eISSN: 2379-6367


Research Article Volume 12 Issue 3
1Faculty of Pharmacy, Al-Quds University, P. O. Box 20002, Abu- Dies, Palestine
2Al-Quds Bard College, Al-Quds University, Abu-Dies, Palestine
3Life Sciences Department, College of Science and Technology, Al-Quds University, Abu-Dies, Palestine
Correspondence: Mutaz Akkawi, Life Sciences Department, College of Science and Technology, Al-Quds University, Abu-Dies, Palestine, Tel ++972-2-526435785
Received: June 22, 2024 | Published: July 5, 2024
Citation: Abu-Lafi S, Said C, Abu-Remeleh Q, et al. Exploring the potency of wild sage (Salvia officinalis): UPLC-PDA-ESi-MS phytochemical profiling and inhibitory activity against β-Hematin. Pharm Pharmacol Int J. 2024;12(3):108‒116. DOI: 10.15406/ppij.2024.12.00441
The current study employed a semi-quantitative approach to investigate the inhibitory effects of water-extracted wild sage (Salvia officinalis) on β-hematin formation in vitro. Spectrophotometric analysis over a nine-day period evaluated the antimalarial efficacy of water and sodium bicarbonate extracts derived from wild sage leaves. Results indicated that bicarbonate extracts exhibited superior in-vitro effectiveness against malaria compared to water extracts. Furthermore, both extracts exhibited a marginal decrease in efficacy over the nine days. Polyphenols present in the water extract of wild sage were analyzed using UPLC coupled to photodiode array and quadrupole mass spectrometry (LC-PDA-ESi-MS). Key compounds identified included Hispidulin-7-glucuronide, Luteolin-7-O-rutinoside, Apigenin-7-O-glucoside, Luteolin-7-O-glucuronide, Rosmarinic acid, Isorhamnetin, Hispidulin, Pectolinarigenin, Epirosmanol, Genkwanin, Carnosol, Carnosic acid, Hesperetin, and Rosmaridiphenol. The diverse array of polyphenols present in the wild sage extracts effectively formed complexes with free heme, thereby preventing the formation of β-hematin, which is crucial for disrupting the plasmodium parasite during its intraerythrocytic stage.
Keywords: wild sage, Salvia officinalis, Malaria, Hemozoin, β-hematin, polyphenols, UPLC-PDA-MS
LC-PDA-ESi-MS, UPLC coupled to photodiode array and quadrupole mass spectrometry in the electrospray ionization mode; UPLC, ultra-performance liquid chromatography; PDA, photodiode array; ESi, electrospray ionization; MS, mass spectrometry; QDa, quadrupole mass analyzer detector; DMSO, dimethyl sulfoxide; NaHCO3, sodium bicarbonate; CQ, chloroquine diphosphate salt; ACN, acetonitrile; FA, formic acid; H2O, water; NaOH, sodium hydroxide; EtOH, ethanol; ACTs, Artemisinin-based combination therapies; UV-Vis, ultraviolet-visible spectroscopy
Malaria, a serious parasitic infection spread by Anopheles mosquitoes, poses a significant worldwide health challenge, leading to acute and potentially life-threatening complications in humans.1 The findings from the World Malaria Report 2023 highlighted a devastating toll of 608,000 deaths attributed to malaria in the year 2022 alone.2 Although this shows a slight decline of 11,000 deaths compared to 2021, it remains notably higher, by 40,000, than the pre-pandemic levels recorded in 2019. In 2022, approximately 249 million documented cases of malaria were recorded worldwide, indicating the extensive prevalence of the disease and emphasizing the substantial strain it imposes on healthcare systems and communities globally. According to the World Malaria Report, Sub-Saharan Africa alone experienced an estimated 580,000 malaria-related deaths in 2022. Young children in Africa encounter significant health hurdles, particularly concerning malaria, with 76% of malaria-related deaths occurring in children under 5 years old, totaling approximately 462,000 deaths in 2022. Moreover, within the WHO African Region, 36% of pregnancies were exposed to malaria, elevating the risk for pregnant women.2
Malaria is caused by Plasmodium parasites transmitted to humans through the bite of infected female Anopheles mosquitoes. The malaria parasite undergoes a complex life cycle involving two primary stages: the liver stage (hepatic stage) and the blood stage (erythrocytic stage). During the erythrocytic stage, red blood cells are invaded and ruptured. The malaria parasite relies on hemoglobin for growth and survival; utilizing its abundant amino acids for protein synthesis.3 However, the digestion of hemoglobin produces toxic-free heme, which is detrimental to the parasite. This process threatens the parasite's survival by causing cellular damage and disrupting crucial biological processes, endangering its life.4
In order to combat the harmful consequences of free heme, the malaria parasite utilizes a detoxifying mechanism that is essential to its survival. The mechanism includes the transformation of heme into a polymer that is called hemozoin which is insoluble and non-toxic to the parasite and does not threaten its life.5 In this way, the parasite can handle the situation and grow without having anything that limits or blocks its development.6 Understanding this mechanism of heme metabolism is critical in the development of antimalarial drugs. This heme detoxification mechanism serves as a target to destroy the parasite and prevent its survival. Synthetic β-hematin polymer derived from ferriprotoporphyrin (IX), is identical to purified natural hemozoin.7 Inhibiting β-hematin is crucial in developing new antimalarial medications Therefore, a candidate drug may function by preventing the formation of the hemozoin, the nontoxic product, and as a result, the free heme molecules that are toxic to the parasite are accumulated which threaten the life of the parasite and its survival.8
The success of drugs like Chloroquine and Artemisinin-Based Combination Therapies (ACTs) in combating the Plasmodium parasite has been overshadowed by the emergence of drug-resistant strains.9 This resistance poses a significant challenge, necessitating innovative therapeutic approaches. Plant extracts are being explored as potential sources for new antimalarial drugs, offering a sustainable and nature-based solution to drug resistance. This shift underscores the importance of multidisciplinary research in the fight against malaria.
Natural sources, particularly polyphenols, have been studied for possible antimalarial properties due to their diverse chemical structures and functions.10 Polyphenols present in plants, including flavonoids and tannins, have antimalarial properties by disrupting the parasite's life cycle and limiting its growth, which has the potential to overcome resistance.11
Plant-based drugs may offer reduced toxicity and side effects compared to synthetic alternatives.12 Natural compounds are often more readily available and accessible than synthetic counterparts. This can facilitate cost-effective drug development and increase the availability of antimalarial treatments, especially in resource-limited settings where malaria is prevalent. Natural compounds frequently contain multiple bioactive constituents, allowing them to interact with several molecular targets within the parasite. This multitarget approach can be valuable in addressing the complexity and adaptability of the malaria parasite, potentially reducing the likelihood of the development of resistance.13
Wild sage (Salvia officinalis) belongs to the Lamiaceae family; it is widely recognized in herbal medicinal and culinary traditions.14 It is high in essential oils, flavonoids, and phenolic acids, and additionally, it is known for its strong antioxidant and anti-inflammatory properties. Due to the high antioxidant activity of sage extract, it helps neutralize harmful free radicals in the body, thereby reducing oxidative stress, and therefore promoting the prevention of cancer and premature aging.15
Sage (Salvia) species, long valued in traditional medicine in Asia and the Middle East, possess diverse pharmacological benefits. They alleviate pain and fight oxidative stress, inflammation, and infections. Recent studies highlight their potential in treating conditions like depression, dementia, obesity, diabetes, heart disease, and cancer.16
The utilization of wild sage in antimalarial drugs represents a fascinating intersection of traditional medicine and modern pharmacology.17 Wild sage, which has a long tradition of being used by Indigenous people to treat different health issues, has become recognized as a valuable source of compounds that possess strong antimalarial properties like polyphenol.10
Researchers have discovered various bioactive substances, including flavonoids and terpenoids that have impressive antimalarial properties. These substances act by focusing on various phases of the malaria parasite's life cycle, which includes preventing its development and multiplication inside the host's blood.18 Moreover, extracts from wild sage have demonstrated promise in fighting drug-resistant strains of malaria, providing a beneficial option in addition to current antimalarial treatments. Utilizing traditional knowledge alongside modern scientific methods is crucial in developing antimalarial drugs from wild sage for effectively tackling global health issues.
Phenolic acids (polyphenols) have been reported to exhibit antioxidant, anticancer, anti-inflammatory, antiviral, and antibiotic effects. However, information on their antimalarial activity is limited. Wild sage has some phenolic acids that may exhibit anti-malarial activity. Examples include caffeic acid, rosmarinic acid, and gallic acid. Studies have shown that rosmarinic acid exhibits various biological activities, including anti-inflammatory, antioxidant, and antimicrobial properties.19 Wild Sage also contains flavonoids such as luteolin, apigenin, and quercetin.20 They contribute to sage's health benefits, and are known for their antioxidant and anti-inflammatory properties as they help protect cells from damage, reduce inflammation, and support heart health.21 Sage has also essential oil which contains α-thujone and β-thujone, which contribute to its aroma and may have antimicrobial properties as well as camphor, which offers anti-inflammatory effects and topical pain relief.22 These compounds suggest sage oil's potential for addressing infections and inflammation.
The objective of this study is to explore the inhibitory impacts of aqueous extracts derived from wild sage on β-hematin, under varying conditions, including the presence or absence of sodium bicarbonate, employing semi-quantitative techniques. Furthermore, we will employ UPLC-PDA-ESi-MS analysis to characterize the bioactive phytochemicals found in the standard aqueous extract of Salvia officinalis.
Dimethyl sulfoxide (DMSO), chloroquine diphosphate salt (CQ) analytical standard, sodium acetate, and hemin chloride, were procured from Sigma-Aldrich. Sodium bicarbonate (99.7-100.3%) was also sourced from Sigma Aldrich. Ethanol (EtOH) was obtained from Merck (Germany), and glacial acetic acid was sourced from Fluka. Wild sage was acquired from a Jerusalem-based local market that specializes in natural plant products.
Preparation of wild sage extract
Two grams of wild sage were immersed in 150ml of distilled hot water at 90 °C, allowed to stand for 20 minutes at room temperature, and subsequently filtered through MN 615.Ø110 mm filter paper. To examine the effect of bicarbonate, 0.5g of NaHCO3 was dissolved in 150 mL of hot water, which was then employed in the extraction procedure. The filtrate was then evaporated using a rotary evaporator under reduced pressure. The resultant extract was lyophilized until a constant weight was attained and then stored in an amber bottle in the refrigerator.
Semi-quantitative in-vitro method
Following Deharo protocol outlined in detail previously, a mixture comprising 50μL of 0.5mg/ml hemin chloride freshly dissolved in dimethyl sulfoxide (DMSO), 100μL of 0.5 M sodium acetate buffer (pH 4.4), and 50μL of the selected water sage extract (different concentrations and conditions were used) was incubated in a standard non-sterile 96-well flat-bottom plate at 37ºC for 18-24 hours. Subsequently, the plate underwent centrifugation, removal of the supernatant, and pH measurement. The wells were then washed with 200μl DMSO per well to eliminate free hemin chloride, followed by another round of centrifugation. The remaining β-hematin was dissolved in 200μl of 0.1 M NaOH for spectrophotometric analysis at 405nm using an ELISA reader.23
Chromatographic UPLC-PDA-ESi-MS analysis
Analysis utilizing Ultra-Performance Liquid Chromatography coupled with Photodiode Array Detector (PDA) and Quadrupole Mass Analyzer Detector (QDa) was performed using the Acquity UPLC H-Class system (Waters, USA) with Empower software. The chromatographic column employed was the Acquity UPLC BEH C18 (50mm x 2.1mm I.D., 1.7μm) alongside the Acquity BEH C18 1.7 μm guard column (Vanguard 2.1 x 5 mm, Waters, USA). Ultrapure water LC-MS grade (Milli-Q-Millipore) and Acetonitrile (ACN) Lichrosolv hypergrade (J.T. Baker), as well as Formic acid (FA) HCOOH, LC-MS grade (Merck), were utilized. The column and sample temperatures were maintained at 35˚C and 25˚C, respectively. The mobile phase consisted of 0.1% formic acid (FA) in water (A) and 0.1% formic acid in acetonitrile (B). The gradient began at 95% A, held for two minutes, and then increased to 95% B over 20 minutes, followed by maintaining 100% B for an additional two minutes. A delay injection time of approximately 7 minutes was allowed to equilibrate the column. The flow rate was set at 0.3 mL/min. The PDA detection range was 210-400 nm, while the mass spectrometry (MS) ionization was conducted in both negative and positive Electrospray Ionization (ESI) modes, with a mass range of 200-1000 Da.
In-vitro semi-quantitative method
The potential in-vitro effectiveness of water and sodium bicarbonate extracts obtained from wild sage leaves against malaria was assessed over a period of nine days by spectrophotometrically analyzing solvated β-hematin at 405 nm. Four different extraction time points were investigated: one, two, three, and nine days prior to measurement. Negative controls consisting of water and sodium bicarbonate solutions were used as benchmarks for comparison with the effects of wild sage leaf extracts. Furthermore, a positive control of chloroquine (CQ) at 0.1 mg/mL was included to validate the assay and serve as a reference for anticipated antimalarial activity. Each data point depicted in the column chart represents the average of 16 separate experiments.
Figures 1–Figure 4 depict a comparative analysis that highlights the antimalarial efficacy of wild sage leaf extracts, revealing significant differences between the water and sodium bicarbonate extraction methods. Specifically, experiments employing sodium bicarbonate extracts demonstrate superior antimalarial effectiveness, evident from the lower absorbance rates of solvated β-hematin. Conversely, experiments conducted without sodium bicarbonate show an increase in absorption rates over time, suggesting ongoing formation of solvated β-hematin and reduced inhibitory effects. Furthermore, the consistent antimalarial effectiveness observed throughout the nine-day duration underscores the potential of wild sage as a promising natural solution for addressing malaria. It is important to note that these results are first of their kind and they will have significance contribution to the malaria research field. This will open the door for further research in this field.
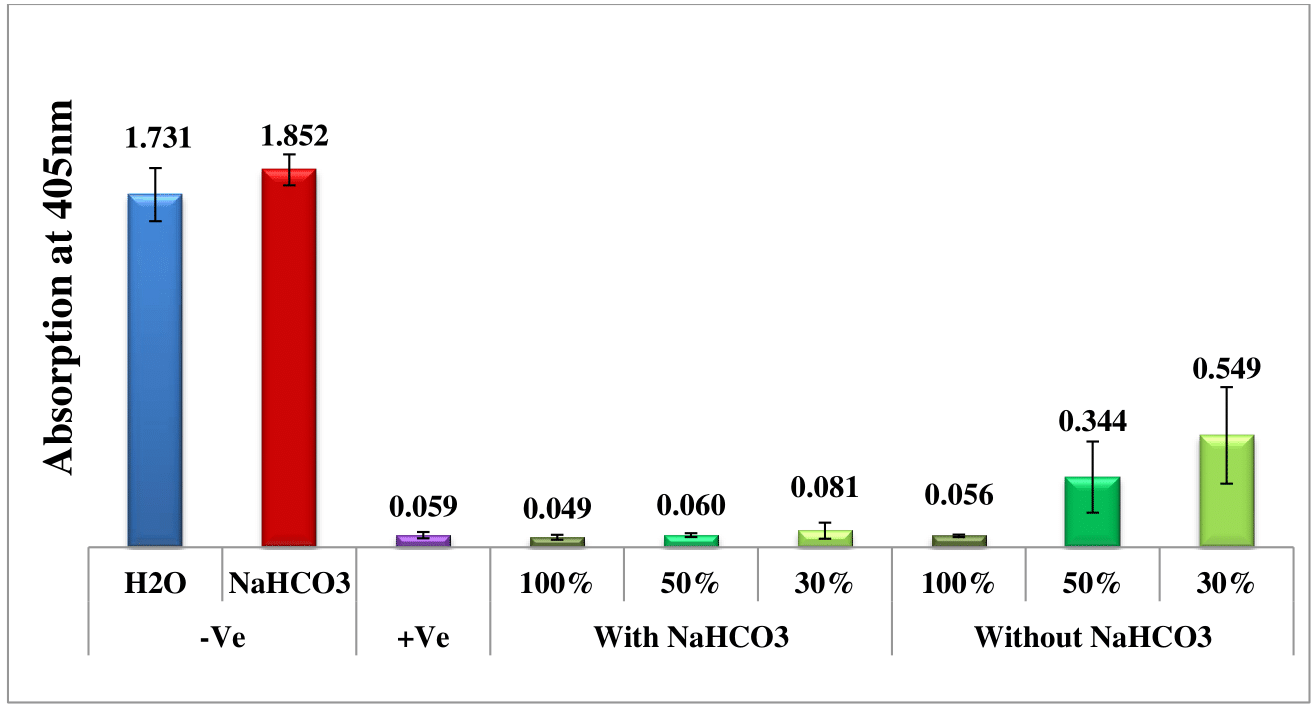
Figure 1 Antimalarial efficacy of extracts from wild sage leaves in comparison to negative (water and sodium bicarbonate) and positive (CQ-0.1 mg/mL) controls, measured by the absorption of solvated β-hematin at 405 nm. The column diagram, prepared one day prior to analysis, presents the results from an average of 16 separate experiments.
UPLC-PDA-ESi-MS analysis of water wild sage extracts
The UPLC-PDA-ESi-MS system was employed to analyze a freshly collected sample of wild sage. Detection was performed using PDA at 330 nm and MS detectors in both positive and negative ESi modes. Figure 5 displays chromatograms detailing the secondary metabolites of polyphenols found in the extract. Table 1 provides a summary of the identified polyphenolic compounds, including their retention times, molecular weights, and multiwavelength maxima. Figure 6 illustrates the chemical structures of the predominant constituents present in wild sage.
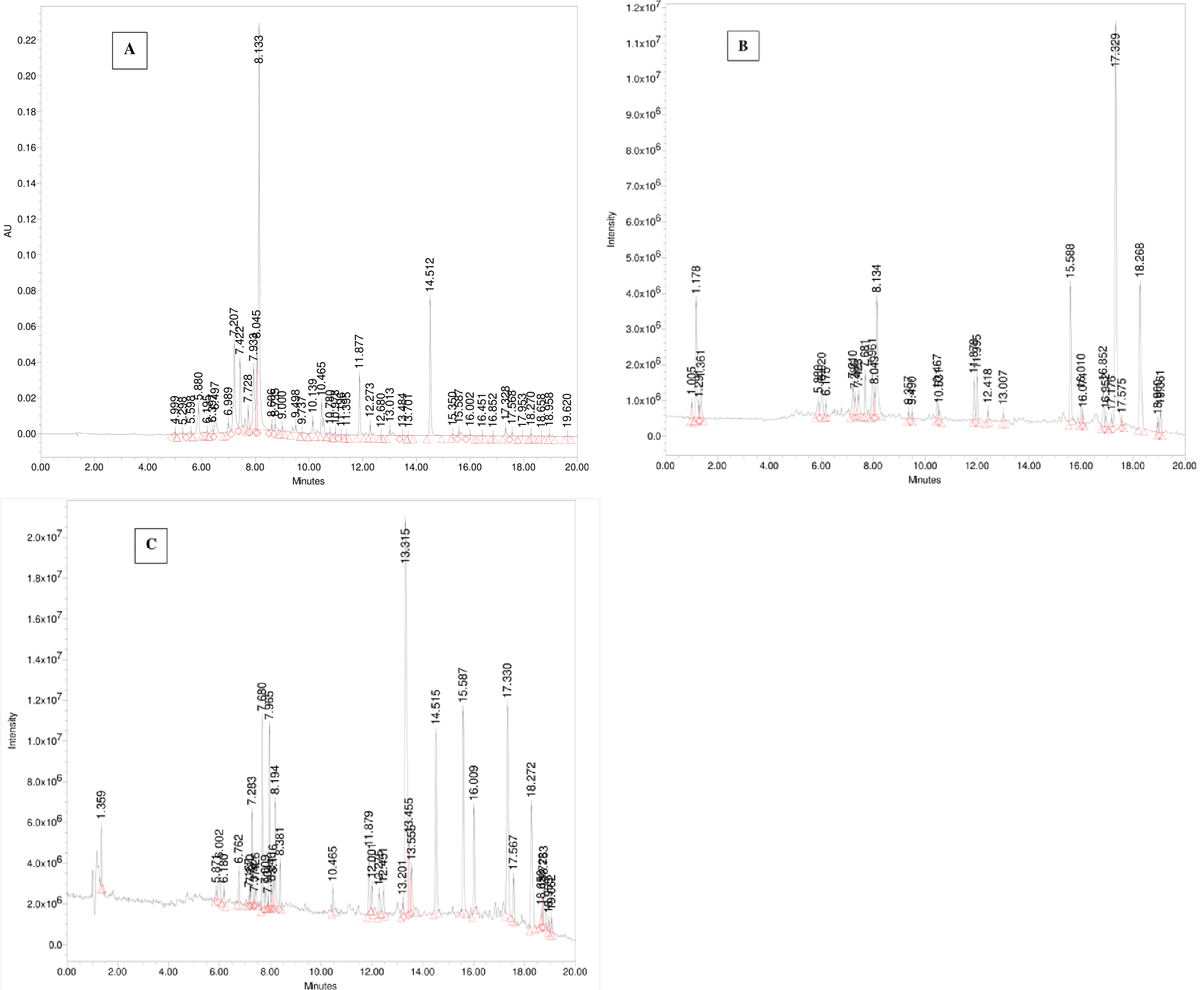
Figure 5 Chromatograms showing polyphenols from a water extract of wild sage were monitored at 330 nm (A), using UPLC in (-)-ESi mode (B), and in (+)-ESi mode (C).
|
# |
Name |
*RT (mins) |
m/z [M-H]- |
m/z [M+H]+ |
UVmax (nm) |
|
1 |
Hispidulin-7-glucuronide |
1.29 |
475 |
||
|
2 |
Luteolin-7-O-rutinoside |
5.88 |
593 |
595 |
270.9, 333.9 |
|
3 |
Apigenin-7-O-glucoside |
6.17 |
431 |
240.2, 327.1 |
|
|
4 |
Luteolin-7-O-glucuronide |
7.21 |
461 |
463 |
253.1, 347.0 |
|
5 |
Rosmarinic acid |
8.13 |
359 |
329.5 |
|
|
6 |
Isorhamnetin |
9.49 |
315 |
317 |
287.5, 328.9 |
|
7 |
Hispidulin |
10.47 |
299 |
301 |
272.7, 334.5 |
|
8 |
Pectolinarigenin |
11.88 |
313 |
315 |
274.6, 333.9 |
|
9 |
Epirosmanol |
11.99 |
345 |
347 |
|
|
10 |
Genkwanin |
13.01 |
283 |
385 |
267.8, 331.4 |
|
11 |
Carnosol |
15.59 |
329 |
331 |
284.4, 322.1 |
|
12 |
Carnosic acid |
17.33 |
331 |
285.1 |
|
|
13 |
Hesperetin |
18.27 |
301 |
283.2 |
|
|
14 |
Rosmaridiphenol |
18.96 |
315 |
317 |
292.5 |
Table 1 Compounds found in wild sage water infusion with their spectral characteristics
*RT: retention time in minutes.
As depicted in Figures 5B and Table 1, the naturally occurring polyphenolic compound, Hispidulin-7-glucuronide ([M-H]¯ m/z 475 Da) eluted at 1.29 minutes. The mass spectrum of Luteolin-7-O-rutinoside showed a typical deprotonated pseudo molecular ion [M-1]¯ of 593 Da and a protonated [M+H]+ ion at m/z 595 Da, eluting at 5.88 minutes (Figure 7). Apigenin-7-O-glucoside was detected at 6.17 minutes with a deprotonated MS peak at m/z of 461 Da. Luteolin-7-O-glucuronide ([M-H]¯ m/z 461 Da, [M+H]+ m/z 463 Da) had an elution time of 7.21 minutes. The dimer adduct [2M-1]¯ with an m/z of 923 Da was observed due to its high concentration (Figure 7).
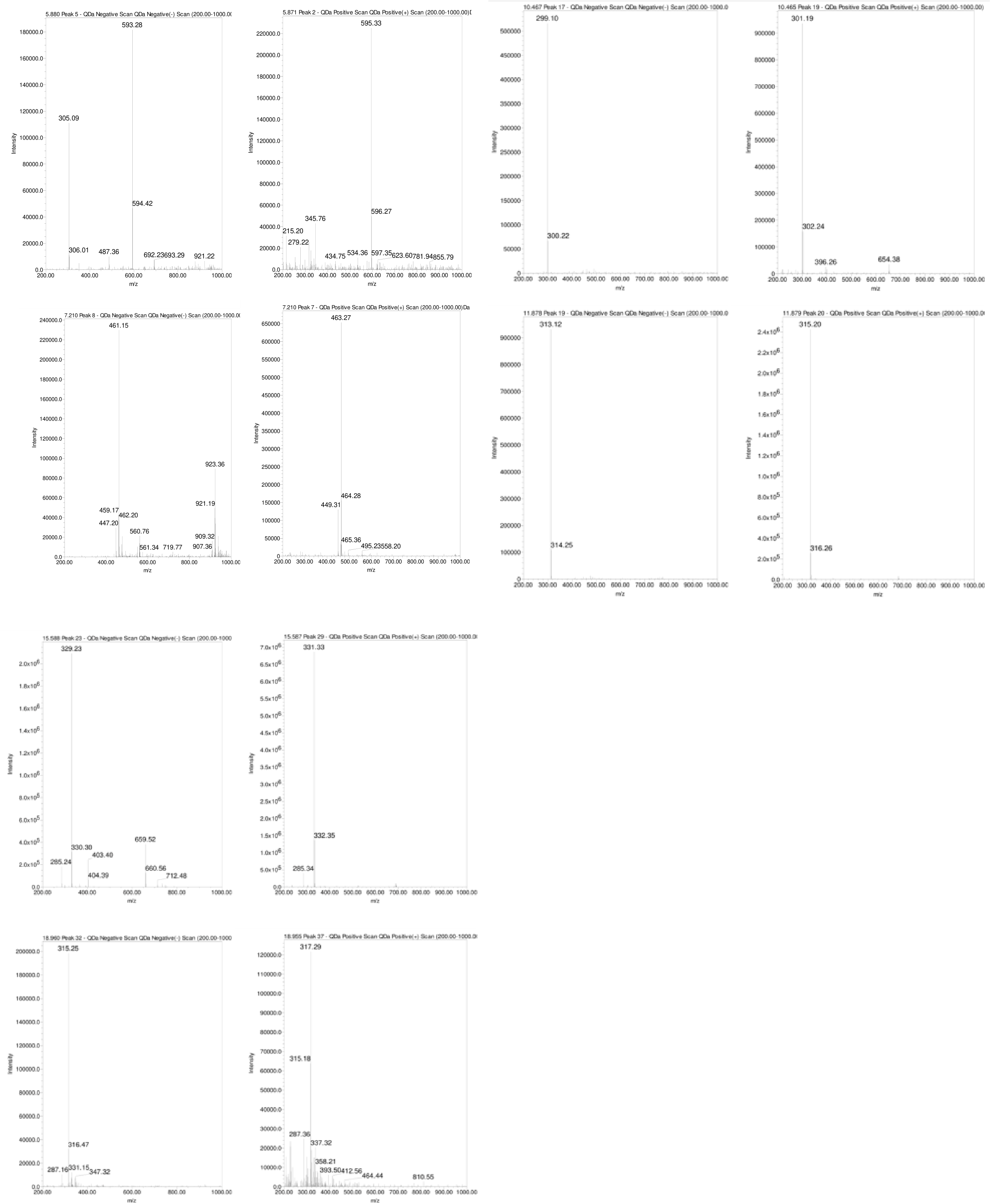
Figure 7 MS of chosen polyphenols present in the water extract of wild sage, utilizing both negative and positive electrospray ionization (ESI) modes.
Rosmarinic acid eluted prominently at 4.484 minutes with a typical m/z of 359 Da in the negative ESi mode. Additionally, successive peaks eluted between 9.49 to 15.59 minutes, corresponding to compounds exhibiting a 2 Dalton difference in their mass-to-charge ratios (m/z) between negative and positive ESi modes, as shown in Figure 7.
Polyphenols-heme complex formation
The primary bioactive phytochemicals in wild sage are characterized by numerous hydroxyl groups and, to a lesser extent, carbonyl and occasionally carboxylic acid groups. Similar observations were made in our previous studies.24–26 These functional groups enable polyphenols to form multiple non-covalent complexes with heme. It is hypothesized that the stable and energetically favorable interaction between these compounds and ferriheme inhibits β-hematin formation. Understanding the stability of these complexes is challenging due to the diverse hydroxyl groups present in such compounds, which adds to their complexity.
It is proposed that the polyphenol-heme complex likely forms through two main interaction mechanisms. The primary interaction is believed to occur between the most complementary hydroxyl groups of the polyphenol and the carbonyl group of the unbound propionic carboxylate heme groups, involving hydrogen bonding. Additionally, hydrophobic π-stacking between the rings of the polyphenol and the porphyrin rings of the heme moiety may also significantly contribute to the underlying mechanism. Identification of the polyphenol-heme complex and elucidation of the intricacies of their interaction could be facilitated by molecular modeling and appropriate docking experiments, providing deeper insights into the facilitation or challenges associated with such bindings.
Water extracts of Salvia officinalis contain polyphenols that exhibit antiplasmodial properties, suggesting their potential as a promising treatment for malaria. Their accessibility, affordability, potency, safety, and ease of preparation further underscore their suitability as a malaria remedy. These findings present opportunities for developing novel and effective antimalarial medications. Adding carbonate to Salvia officinalis tea is recommended for optimal efficacy.
None.
The authors declare that there is no conflict of interest.

©2024 Abu-Lafi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.