eISSN: 2379-6367


Research Article Volume 4 Issue 5
1Govt. Motilal Vigyan Mahavidyalaya, India
2MK Ponda College, Barkatulla University, India
Correspondence: Manju Tembhre, MK Ponda College, Barkatulla University, Bhopal- 462038, MP, India
Received: June 05, 2016 | Published: September 30, 2016
Citation: Ahirwar S, Tembhre M. Assessment of acetylcholinestrase inhibiton by Bacopa monneiri and acephate in hippocampus of chick brain for impediment of Alzheimer’s disease. Pharm Pharmacol Int J. 2016;4(5):413-417. DOI: 10.15406/ppij.2016.04.00088
Introduction: In Alzheimer’s disease (AD), a depletion of the neurotransmitter, acetylcholine (ACh) occurs due to the destruction of cholinergic neurons. By inhibiting Acethylcholinesterase (AChE), the enzyme which catalyses break down of ACh, levels of this neurotransmitter can be elevated and function may be improved.
Objective: This study sought to find out whether the Acethylcholinesterase (AChE) activity, enzyme kinetics and histology in the hippocampus of Chick brain were affected in a selective manner by a herbal extract, andorganophosphate Pesticide and combination of both:
Method: AChE activity was determined by Ellman’s method and AChE kinetics by LB plots.
Result: AChE activities due to Bacopa monneiri, acephate and combination of bothin hippocampus of treated chicks were significantly inhibited to 72%, 70.9% and 94% respectively. Our kinetic study reveals that acephate is competitive while Bacopa monneiriwas competitive-noncompetitive inhibitorof AChE. Slight histological variation was observed as the concentration of cytoplasm of CA1 and CA3 pyramidal cell layers in the Hippocampus of chick treated with acephate and ethanolic extract of Bocopa monnieri.
Conclusion: Common herb Bacopa monnerimay help to improve ACh level, but scientific evidence to prove that it can treat Alzheimer's disease, is still to confirm.
Keywords: hippocampus, chick brain, ache, inhibition, kinetics, km, vmax, Bacopa Monneri, acephate
IAEC, institutional animal ethics committee; AD, alzheimer’s disease; Ache, acetylcholinesterase; DTNB, dithionitrobenzoic acid; OD, optical density
World Alzheimer Report of 20151 has estimated that 46.8million people are currently living with dementia worldwide which will be further increased to 74.7million by 2030 and 131.5million by 2050. Alzheimer’s disease (AD) is a neuro-degenerative disorder and common form of dementia, which progress gradually with the age. The common symptom of AD is memory impairment and cognitive shortfall due to low levels of acetylcholine in the brain of AD patients. According to the cholinergic theory, the inhibition of acetylcholinesterase (AChE), an enzyme that catalyzes acetylcholine hydrolysis, increases the levels of acetylcholine in the brain, thus improving cholinergic functions in AD patients.2 At present tacrine, donezepil, rivastigmine and galanthamine are few approved drugs for the symptomatic treatment of Alzheimer’s disease. The clinical response of these drugs has limited effectiveness and has some kind of side effect in AD patients. These side effects include Dizziness and syncope, Bradycardia, atrial arrhythmias, myocardial infarction, angina, seizures Sino-atrial and atrioventricular block.3 Galanthamine is a natural alkaloid first obtained from Galanthus spp. was approved by US-FDA in 2001. Recently Huperzine A, an alkaloid derived from Huperzia spp., has been recommended as commercialized AChEi as a dietary supplement to treat AD symptoms in China, which gives promising results in memory improvement.4 Screening of 23 pure Amaryllidaceae alkaloids and 26 extracts from different species of the genus Narcissus have shown positive results for their Acethylcholinesterase inhibitory activity using galanthamine as a reference.5 Herbal remedies for Alzheimer’s disease have become more and more popular in the recent years and not without a reason that there is a possibility to slow down the brain’s degeneration caused by Alzheimer’s with natural treatments and it has drawn the attention of the scientific community.6 Thus, there is need to develop targeted effective therapeutics for the treatment of Alzheimer’s disease with least or no side effects. OP pesticides act by their ability to inhibit AChE in vertebrates and invertebrates.7‒9 Kinetic analysis of different species: mammalian (monkey, rat, and guinea pig), avian (chicken), piscine (catfish), and amphibian (frog) in brain Acethylcholinesterase have shown differential sensitivity to organophosphate insecticides.10‒12
Bacopa monnieri is a perennial, creeping herb and commonly used nervine tonic and memory enhance herb. It has been used in the Ayurvedic system of medicine for centuries. Acephate is widely used organophosphate pesticide. Acephate kills insects by disrupting their nervous function. This paper reviews the AChE inhibitory effects of a commonly used medicinal herb Bacopa monneri in hippocampus of brain of chick for the treatment of Alzheimer’s disease and dementia and it was compared with acephate, which is potent AChE OP inhibitor. Animal studies have suggested that early stress is associated with alterations in the hippocampus, a brain area that plays a crucial role in learning and memory. Therefore, purpose of this study was to measure both AChE inhibitions, AChE inhibitory kinetics and alterations in hippocampus structure in chick brain due to herb Bacopa monneri and acephate.
Preparation of plant extract
Fresh Bacopa monnieri were procured from Jawaharlal Nehru Agricultural University, Jabalpur (M.P.) India. Plant material was validated by botanist. Leaves and twigs were cut and carefully washed in water; dried in shade. Dry plant material was ground to powder. Ethanol extract of Bacopa monnieri was prepared in Soxhlet apparatus using 90% alcohol at 75-80˚C. Filtrate was dried to semisolid mass, freeze-dried and re-constituted in de-ionized water prior to assy.
Animals and treatment
20 chicks of 100-125g were divided into 4 groups and each group consisted of a minimum of five animals. Separate animals were used for each experiment. They were housed separately in polypropylene cages, maintained in laboratory condition at 30˚C with 12/12hours light and dark cycle for 10days. They were allowed free access to commercial feed and water ad libitum. Ethical clearance for the use of animals was obtained from the Institutional Animal Ethics Committee (IAEC). The animals were orally dosed via feeding needle as: Group-I: Ground nut oil 1ml/kg b.w. for 4days, served as control (vehicle); Group-II: 37.8mg/kg b.w. acephate (1/25th of LD50) in ground nut oil daily for 4days; Group-III : Ethanol extract of Bacopa monneiri 100mg/kg in deionized water for 7days; Group-IV: Pretreatment of ethanol extract of Bacopa monneri (100mg/1kg b.w.) for 7days daily followed by 37.8mg/kg b.w. acephate for 4days.
Tissue preparation
At the end of experiment, animals were euthanized and brains were dissected out according to Zeman and Innes.13 Hippocampus from each chick was quickly isolated, weighed and thoroughly washed with isotonic saline. Tissues were homogenized in buffer (7.4 pH) followed by centrifugation at 40C and supernatant was used as source of enzyme.
Estimation of AChE activity
The hippocampus AChE activity was measured using the Ellman’s method.14 The end point was the formation of the yellow color because of the reaction of thiocholine with di-thio-bis-nitrobenzoate ions. The rate of formation of thiocholine from acetylcholine iodide in the presence of tissue cholinesterase was measured using a SL164 UV-VIS spectrophotometer. The sample was first treated with 5,50 -dithionitrobenzoic acid (DTNB), and the optical density (OD) of the yellow color compound formed during the reaction at 412nm every minute for a period of 2min was measured. All measurements were done in duplicate. Specific activity was expressed in μmol/min/mg protein.
Protein determination
Protein was determined by the Lowry method15 using BSA as the standard. Aliquots of homogenate were diluted with reagents then 0.5ml Folin’s reagent was added and kept for incubation for 20min then the colour was read at 620nm against a reagent blank. Measurements were done in duplicate.
AChE kinetics
Kinetic parameters like Km and Vmax were calculated by Line weaver-Burk plots, which were drawn from assays using acephate (37.8mg/kg) and Bacopa monneiri extract (100mg/kg) at various substrate concentrations (0.66mM, 0.44mM, 0.33mM, 0.26mM ATCI). From these Kinetic parameters (Km & Vmax) were determined for each assay by plotting the reciprocals of velocity and substrate concentrations.
Histological examination
Tissues from each group of chicks were fixed in aqueous Bouin’s fluid, dehydrated and embedded in paraffin wax. Serial sections were cut at 7 micron and stained with Haematoxylin and Eosin. The microphotographs of serial sections were taken at 400X with computer-aided microscope (Leica).
Statistical analysis
For the data of statistical comparison between different treatments and control groups, data were analyzed by Student’s t-test to determine the effect of the treatment. The level for the accepted statistical significance was P>0.05.
A few studies have been undertaken to evaluate AChE inhibitory potential herbs and pesticides in different discrete regions of Brain of chick.16‒19 Present investigation is aimed at the assessment of in vivo AChE inhibition, its kinetics and histological alterations in hippocampus of brain of chick subjected to intoxication with Acephate, treatment with Bacopa monnieri singly and in combination with acephate. Our result showed that AChE specific activity in hippocampus of control chick was 10.58±8.4x10-6 M. However, daily oral dose of acephate (34mg/kg b. w.) for fourdays to chick produced 70.9% significant AChE inhibition (Table 1). Organophosphorus insecticides Chlorpyrifos and methyl parathion were potent inhibitor of Acethylcholinesterase in hippocampus.20 Hippocampus has been reported to sensitive to AChE inhibition by multiple exposures to 2-organophosphate pesticides i.e. Tri-Ortho-Toly phosphate and Chlorpyrifos21 and Chlorpyrifos and methyl parathion.20
Parameters |
Control |
Acephate |
Bacopa Monnieri |
Bacopa monnieri and Acephate |
AChE specific activity |
10.58 ± 8.2 |
3.49 ± 2.1*** |
2.93 ± 0.07 |
0.619 ± 0.203* |
AChE inhibition % |
- |
70.9 |
72 |
94 |
Km x 10-3 M |
0.25±0.15 |
0.83±0.35*+232% |
0.37±0.25***+48% |
0.44±0.26**+76% |
Vmax (A / min/mg protein) |
0.12 |
0.12 |
0.11 |
0.17 |
Table 1 Acethylcholinesterase activity (Activity/mg protein/min.), % inhibition, Km x 10-3 and Vmax of AChE of Hippocampus of Chick brain treated with sub lethal dose of Acephate (34 mg/kg b. w.), Bacopa monnieri (100 mg/kg b. w.) and pretreatment of Bacopa monnieri (100 mg/kg b. w.) Followed by Acephate (34 mg/kg b.w.). The AChE specific activity is expressed in µ moles of ATCI hydrolyzed / mg protein /min
Values are expressed as mean ± S.D. of 5 individual animals in each group analyzed by student’s t-test P < 0.01*; P < 0.02**; P < 0.05***, compared with control vs. treated.
Oral dose of 100mg/kg b. w ethanol extract of Bacopa monnieri yields 72 % AChE inhibition in hippocampus (Table 1). However, AChE inhibition increased to many fold i.e. 94% after 7days pretreatment of of Bocopa monnieri ethanolic extracts (100mg/kg b. w.) followed by acephate (34mg/kg b.w.) daily for 4days. The hydroalcohol extract from Centella asiatica, Nardostachys jatamansi, Myristica fragrans, Evalvulus alsinoides inhibited 50% of AChE activity.22 The Chinese herb Huperzia serrata has been shown to possess antioxidant and neuroprotective properties suggesting thereby its potential in the treatment of Alzheimer’s disease.4 Huperzine A is a medicinally active plant derivative found in Huperzia serrata. This drug has been shown to inhibit acetylcholinesterase (such as tacrine and donepezil) and to improve memory and mental functioning in patients with Alzheimer’s and other severe conditions.23 Three herbal plants, Melissa officinalis, Salvia officinalis & Rosmarinus officinalis have proved their efficacy in the management of patients with AD as they are active not only in inhibition of AChE or β-amyloid deposits inhibition in-vitro.24,25 Current treatments option available for dementia are very limited (cholinesterase inhibitors, NMDA receptor antagonist)26 they only provide short term symptomatic relief and do not prevent disease progression. Also, over the last decade a large number of drug candidates have failed in clinical trials.27
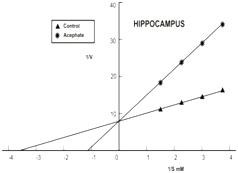
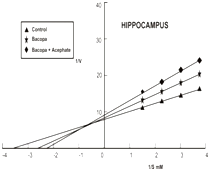
The primary mechanism of action of organophosphate pesticides and anticholinesterase herbs includes the inhibition of AChE kinetics.10,28,29 In the present study the inhibitory Kinetics of AChE was determined by applying Linewear-Burk plot. The kinetic parameters Michaelis Menten Constant (Km) and maximum velocity (Vmax) were calculated in control and treated groups to compare inhibitory kinetic in hippocampus of chick. The km was determined by slops for the control and acephate inhibited AChE, intersecting separately on the abscissa represent the Km value and the slops intersecting on the ordinate indicate Vmax values. Data of our kinetic study also substantiate AChE inhibition in hippocampus of chick. The km value in hippocampus of control group was 0.25±0.15mM, which significantly increased with treatment of acephate (0.83±0.35mM) and Bocopa monnieri (0.37±0.25mM). The Km also elevated to 0.44±0.26 in the mixed treatment group IV (Figure 1) (Table 1). It was interesting to note that acephate and Bacopa monnieri display different nature of AChE inhibition in hippocampus. This was apparent as the Vmax values were decreased while Km increased in chicks after treatment with Bacopa monnieri singly and in combination with acephate as compared to untreated groups of chick. Thus, Bacopa monnieri yields mixed AChE inhibition i.e. competitive-non competitive (Figure 1) (Table 1). Such type of mixed inhibition kinetics is typical of some medicinal plants; due to the great variety of compounds they contain all acting in different ways.30 Contrary to this, acephate produced competitive AChE inhibition indicating increasing Km in treated chicks and constant Vmax in both control and acephate treated chicks (Figure 1) (Table 1). Abdulaziz Al-Jafari31,32 reported Cyclophosphamide reversibly inhibits AChE activity of Chicken brain.
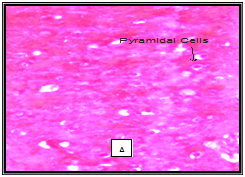
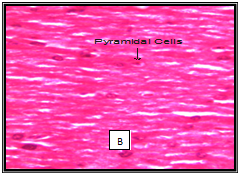
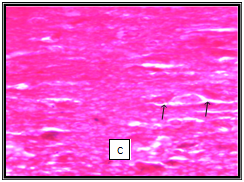
In our study a few histological variations was observed in the Hippocampus showing concentration of cytoplasm of CA1 and CA3 layers in chick treated with acephate and combined treatment group. No significant changes were observed in hippocampus structure in chick treated with Bacopa monnieri. (Figure 2). It has been reported that Bacopa monnieri in rat hippocampus significantly increased the density of cholinergic neurons in hippocampus.32 Sarin and soman affected adversely pyramidal cells of CA1 and CA3 in the hippocampus.33 Similar study on the toxicity of alpha-cypermethrin at the dose of 250mg/kg b. w. for four weeks showed focal concentration in CA3 layer of hippocampus.34 Other investigation on Chlorpyrifos and Cypermethrin intoxication resulted in increased density of the cytoplasm in neurocytes of hippocampus.35 Shih et al.36 opined that critical histological examination of brain sections is essential in determining the nature and extent of such changes to understand relationship of the severity of cellular injury and degree and duration of toxicant-induced convulsions. Our findings clearly show that Bacopa monnieri is potent inhibitor AChE as OP pesticide acephate does. Kinetic study confirms its mixed nature of inhibition. It does not produce any adverse effect on histology of hippocampus.

Since, AChE inhibition plays a critical role in slowing progression of AD; it seems that Bacopa monnieri could act as anti-Alzheimer with at least three mechanisms: AChE inhibition, antioxidant and anti-inflammatory activity. It is a potential cognitive enhancer and neuroprotectant against Alzheimer's disease.32,37 This approach has a particular success as these cholinergic neurons are found mainly in regions associated with learning and memory - spreading from the basal forebrain38 and hippocampus39 up to the cerebral cortex.40 In order to find the mechanisms more investigations are necessary. Further works on Bacopa monnieri could lead to isolate bioactive compounds helpful in AD. The paucity of treatment is due to the multi-factorial nature of AD associated cognitive dysfunction. There is a need to develop combination therapies, however adverse effects associated with modern synthetic drugs could prove to be prohibitive when trying to develop them as combination treatments.
None.
Author declares that there is no conflict of interest.

©2016 Ahirwar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.