eISSN: 2379-6367


Review Article Volume 13 Issue 1
1Worcester Academy, 81 Providence St, Worcester, MA, 01604, USA
2Heal Africa Hospital 111 Lyn Lusi, Goma, North Kivu; D.R. Congo
Correspondence: Pascal Gisenya, Heal Africa, Hospital 111 Lyn Lusi, Goma, North Kivu, D.R. Congo, Tel +1 587 9747611
Received: February 01, 2025 | Published: February 13, 2025
Citation: Wang W, Gisenya P. Artemisia Afra and Artemisia Annua against pulmonary tuberculosis. Pharm Pharmacol Int J. 2025;13(1):21-24. DOI: 10.15406/ppij.2025.13.00460
Pulmonary tuberculosis (TB), caused by Mycobacterium tuberculosis, remains a major global health challenge. The increasing incidence of multidrug-resistant TB (MDR-TB) necessitates the exploration of alternative therapeutic options. Artemisia afra and Artemisia annua are two medicinal plants used historically in traditional medicine to treat various ailments, including respiratory diseases. Researchers have discovered that extracts found in the two plants, such as artemisinin and certain flavonoids and flavones, have significant anti-TB effects. This review evaluates the potential of Artemisia Afra and Artemisia Annua in treating pulmonary tuberculosis, focusing on their pharmacological properties, preclinical, clinical studies, and their potential integration into TB treatment regimens.
Keywords: translational medical sciences, disease treatment and therapies, tuberculosis, Artemisia Afra, Artemisia Annua
Pulmonary Tuberculosis is a contagious infection of the lungs caused by the bacterium Mycobacterium tuberculosis (MtB). A victim of the infection experiences symptoms such as prolonged cough, chest pain, weakness or fatigue, weight loss, fever, night sweats. While not as prevalent in Western countries, the infection is abundant in many developing nations, making it a major global issue. The World Health Organization (WHO) estimated that in 2022 globally, a total of 10.6 million were infected with the disease and 1.3 million died from it, making it the second leading infectious cause of death after COVID-19.1
While there are treatments for pulmonary tuberculosis, one of the biggest challenges is the growth of multi-drug resistance to these treatments, with around 410,000 cases of drug resistance in 2022, which has made the issue difficult to manage efficiently and adequately.1 Standard pulmonary tuberculosis treatment recommends regular antibiotics such as isoniazid and rifampin, with the duration of these medicines taking up to usually several months. However, factors such as inappropriate medications and irregular treatments, like missed doses due to unpleasant side effects, the bacterial fitness of the tuberculosis bacterium allows itself to adapt its metabolism to survive in a particular environment.
As a result, cases of resistance to first-line drugs such as rifampicin and isoniazid have surged. Those diagnosed with multidrug-resistant tuberculosis (MDR-TB) have to turn to second-line treatments. These second-line treatments are not only much more expensive and have more side effects compared to first-line treatment, but the treatment can also take as long as 24 months. Due to its inefficiency, toxicity, and expense, this line of treatment has an abysmal success rate of only 54%. As a result of these disadvantages, only 32% of the United Nation’s target of 1.5 million people, or around 500,000 people, enrolled for treatment for MDR-TB from 2018 to 2020. Therefore, there is an urgent need to look for alternative treatments for tuberculosis.1–4
Artemisia annua (A. annua), commonly known as sweet wormwood, respectively, has been used in traditional medicine, mainly as antimalarial medicinal plants. In 2015, the Nobel Prize in Physiology or Medicine was awarded to Chinese scientist Youyou Tu for her groundbreaking discovery of artemisinin,5,6 an active antimalarial ingredient derived from the plant A. annua. Recent research,7–9 has unveiled additional medicinal properties of the Artemisia plant, including antiviral activity and antibacterial effects. Artemisia annua have subsequently gained attention as a potential alternative treatment for MDR-TB.7–9
Recent studies have also highlighted that Artemisia afra (A. Afria), commonly known as African wormwood, a traditional medicinal plant, and exhibits similar therapeutic properties as A. annua, particularly, its effectiveness against tuberculosis may even match or surpass A annua.10–15 This review aims to assess the current research on the efficacy of the A. annua and A. Afria and against pulmonary tuberculosis.
Pharmacological properties of A. afra and A. annua
Macroscopic properties (Figure 1)
Artemisia belongs to the daisy family Asteraceae, which has almost 500 species. A. annua and A. afra are two of the most well-studied species.
Chemical composition
Unlike A. annua, the definite active ingredient has not been determined yet in A. Afra. More than 20 known bioactive compounds have been identified, including flavonoids, davanone, scopoletin, caffeoylquinic acid, etc.9–15,17 Although A. afra and A. annua are all belong to Artemisia family, A. afra has two significant features. First, only trace amounts or none of the artemisinin can be found in the A. afra. Second, A. afra has a significantly higher flavonoid content. For example, the flavonoid luteolin in A. afra reaches concentrations as high as 1.9 mg/ g. Compared to less than 0.2 mg/g in A. annua. Flavonoids, a class of abundant plant phenolic compounds, are known for their diverse biological properties, including Antioxidant, Anti-inflammatory, Anticancer and antimicrobial activities, with over 6000 identified to date.
Notably, a study by Lehane et al.,18 demonstrated that common dietary flavonoids inhibit the growth of the intraerythrocytic malaria parasite. Additionally, Coutinho et al. reported that flavonoid-containing drugs exhibit activity against malaria in mice and show efficacy in vitro against chloroquine-resistant Plasmodium falciparum.19 This suggests that high flavonoids luteolin in A. afra may play a pivotal role in its potent antiplasmodial activity and could be one of the key compounds contributing to its therapeutic efficacy.
Anti-tuberculosis activity
Mycobacterium tuberculosis can sense oxygen levels and become dormant when the oxygen level is lower. This dormancy allows the bacteria to survive under the stress of low-oxygen environments. When the MtB reaches the dormant stage, antibiotics and other treatments are often ineffective. This resistance, due to the inability of the drugs to kill the bacteria in their dormant state, is one of the most important mechanisms that make Mtb resistant to anti-TB drugs.
The addition of artemisinin has been shown to prevent MtB from entering a dormant state. Abramovitch,20 screened 540,000 compounds and identified that Artemisinin targets the bacterial oxygen sensor theme, disrupting its ability to sense the amount of oxygen. This mechanism provides a theoretical foundation for the potential of artemisinin in combating MDR-TB. The finding support its use as a complementary treatment alongside other anti-tuberculosis drugs.
Patel et al., observed synergistic efficacy of artemisinin when combined with rifampicin in an in vitro study.21 The combination significantly induced peroxide production in a concentration dependent manner, with higher levels of peroxides observed in cells treated with this drug pair. These elevated peroxide levels contributed to bacterial membrane disintegration, facilitating the clearance of bacterial cultures. The study also noted that other common anti-tuberculosis drugs, such as isoniazid, exhibited similar synergistic effects when paired with artemisinin. Given the unique structure of the endoperoxide bridge, the researchers emphasized that the efficiency of artemisinin in treating tuberculosis primarily stemmed from its ability to generate peroxides, which are highly effective against bacteria. The addition of ascorbic acid, an antioxidant, reduced peroxide production, thereby diminishing the antibacterial effects of artemisinin, underscoring the crucial role of peroxides in its mechanism of action.9
Choi et al.,22 investigated the anti-tuberculosis potential of artesunate, a derivative of artemisinin, through both in vitro and in vivo studies. Artesunate, a water-soluble sodium hemisuccinyl ester of dihydroartemisinin, was evaluated using the resazurin microtiter assay, along with artemisinin and first-line antituberculosis drugs, across concentrations ranging from 9.375 to 300 µg/mL to determine their inhibitory effects. After five days, both artesunate and artemisinin demonstrated significant bacterial inhibition at a concentration of 75 µg/mL. There was no notable difference in anti-tuberculosis activity between the two compounds. To further assess efficacy, the Mycobacteria Growth Indicator Tube (MGIT) MGIT 960 system assay was employed. In this setup, bacteria were exposed to concentrations ranging from 37.5 to 600 µg/mL of artesunate or artemisinin. Artesunate demonstrated superior efficacy, as bacterial growth in the presence of 300 µg/mL of artesunate was delayed until day 15 and ceased entirely by day 21. In contrast, bacteria treated with 600 µg/ mL of artemisinin began growing on day 12.6. The minimum inhibitory concentration (MIC) for artesunate was calculated at 300 µg/mL, compared to 600 µg/mL for artemisinin, confirming artesunate's stronger inhibitory effect. In vivo studies further supported artesunate’s superior efficacy. Female rats infected with tuberculosis were treated with 3.5 mg/kg of artesunate or artemisinin daily for four weeks. Lung extractions revealed significantly lower tuberculosis colony counts in the artesunate-treated group compared to those treated with artemisinin.23–25
Therefore, Choi concluded that artesunate exhibits stronger anti-tuberculosis activity than artemisinin, making it a promising candidate for further development as an anti-tuberculosis agent.
Weather P et al., investigated the bactericidal effects of dichloromethane extracts from A annua and A afra on Mycobacterium tuberculosis (Mtb) strain mc26230 under hypoxia and with various nutritional carbon sources (glycerol, glucose, and cholesterol) that mimic a condition frequently encountered by Mtb during human disease.25 Both extracts exhibited significant bactericidal activity against Mtb across all tested carbon sources and retained their efficacy under hypoxic conditions. Notably, A. afra demonstrated the most consistent bactericidal activity across all carbon sources, whereas A. annua exhibited its highest activity only with glycerol as the carbon source. These findings highlight the therapeutic potential of both extracts in combating drug-resistant Mtb, with A. afra emerging as a potentially superior candidate.
Weather P et al., further identified and characterized a methoxylated flavone compound, C17H14O5, or 5-hydroxy-4’,7-dimethoxyflavone (Figure 3) that is mainly responsible for anti-Mtb effect in A. afra extracts. 5-hydroxy-4’,7-dimethoxyflavone belongs to flavonoids (subclass 1 flavones) (https://phyproof.phytolab.com/en/reference-substances/details/5-hydroxy-4-7-dimethoxyflavone-85750).
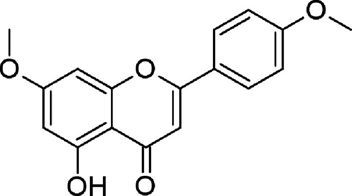
Figure 3 Bond-line structure of 5-hydroxy-4’,7-dimethoxyflavone24
This particular flavone is extremely rich in A. afra leaf with a concentration of as high as 2.29 mg/g. The activity of 5-hydroxy-4’,7-dimethoxyflavone against Mtb under hypoxia-induced growth arrest was so potent that, at a concentration of 312.5 µg/mL, it was able to kill a colony of the in less than a week; at concentration of 0.3125 mg/mL, no colonies were observed after two days of treatment.
Buwa, et al.,26 also showed that A. afra is very effective against TB in vitro. Ntutela S, et al.,27 reported A. afra extract kill MtB in a dose-dependent manner in vitro with IC50=260-290 microg/mL, and the specific extract’s fraction containing several sesquiterpene lactones (this is found in Artemisia afra) demonstrated even more significant antibacterial activity having IC50=1.9 microg/mL.
Safety and toxicity studies
The safety profiles of A. annua and A. afra are well-documented, with minimal toxicity reported. Choi,22 conducted in vivo studies on mice using artemisinin and artesunate, revealing no significant declines in the animals' health. Mekonen K, et al.,28 further investigated the acute and sub-acute toxicity of A. afra in mice, demonstrating that the lethal dose 50 (LD50) exceeded 5000 mg/kg during acute testing. Even when varying doses were administered daily in sub-acute studies, no damage was observed in critical organs such as the brain, heart, or adrenal glands.
Similarly, Kane, et al.,29 found that extremely high dose of A. afra extract were safe in mice, with only one death out of 30 mice at 2500 mg/kg and no adverse effects on organ health or weight.
Additionally, a collaborative study by WHO scientists from 9 research institutions reported a study showed that a combining of A. afra and A. annua produced synergistic effects in the killing of Plasmodium falciparum raising the possibility of similar synergistic benefits for TB treatment when combined with standard drugs like isoniazid and rifampin.30
Although clinical data on the use of A. annua and A. afra in TB treatment remain limited, emerging studies are promising. Dr. Pascal Gisenya and his team 31 from HEAL Africa conducted an empirical study on 25 pulmonary TB patients with drug resistance in the Congo. Over 30 days, patients received A. afra infusions alongside second-line TB treatments. Symptoms such as fever, cough, and weight loss disappeared, and sputum samples tested negative for TB by the end of the treatment. In contrast, patients on second-line treatments alone showed persistent symptoms and positive sputum tests. Dr. Gisenya’s team also reported two successful case studies. In one instance, a 16-year-old girl named Ester, diagnosed with MDR-TB, tested negative for TB after 33 days of A. afra infusions combined with standard and second-line treatments. Similarly, a 24-year-old man named Jonathan achieved negative TB results within 27 days using the same approach. These outcomes are notable, as standard TB treatments typically require months or even years to resolve symptoms.31
Both A. annua and A. afra have demonstrated significant antibacterial activity in both in vitro and in vivo, suggesting a high potential for of their incorporation into modern TB treatment regimens. These plants offer two advantages: availability and low cost. Artemisia species are easily cultivable in many regions worldwide, making them accessibile to resource-limited populations where multidrug-resistant tuberculosis is most prevalent. The affordability of Artemisia-based treatments further enhances their feasibility compared to expensive first-line drugs.
Despite these encouraging findings, the primary challenge to integrating A. annua and A. afra into TB treatment is the lack of large-scale clinical evidence. Current studies are limited to small cohorts, necessitating rigorous, well-designed clinical trials to confirm efficacy and establish treatment protocols.
As a potential adjunct or alternative treatment, particularly for MDR-TB, A. annua and A. afra show promise in reducing treatment duration and improving outcomes. However, further research is crucial to validate their role in combating TB and overcoming the global burden of drug-resistant strains.
None.
The authors declare that there is no conflict of interest.
None.

©2025 Wang, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.