eISSN: 2379-6367


Research Article Volume 7 Issue 1
1Assistant professor and head, Division of Pharmacology, Mahatma Gandhi University, India
2Research scholar, Division of Pharmacology, Mahatma Gandhi University, India
Correspondence: Sibi P Ittiyavirah, Assistant professor and head, Division of Pharmacology, DPS, RIMSR, Mahatma Gandhi University, Puthupally, Kottayam, Kerala, India, Tel 9446883809
Received: October 28, 2016 | Published: January 31, 2019
Citation: Ittiyavirah SP, Hema S. Ameliorating demyelination effect of curqfen® in cuprizone induced mice model of multiple sclerosis. Pharm Pharmacol Int J. 2019;7(1):17-20. DOI: 10.15406/ppij.2019.07.00227
Multiple sclerosis (MS) is an inflammatory, autoimmune, demyelinating disease of the central nervous system. It normally strikes at an early age, most often the early adult years. Its most frequent symptoms include numbness, impaired vision, loss of balance, weakness, bladder dysfunction, and psychological changes. Curcumin is a potent modulator of microglial gene expression and migration.Rapid metabolism and elimination of curcumin due to the poor bioavailability is the main problem for treating neuro degenerative diseases. CurQfen® is an enhanced bioavailable form of curcumin and a unique formulation of curcuminoids from turmeric along with galactomannas from fenugreek to enhance bioavailability.In this study CurQfen® was used as the test drug of dosage 200 mg/kg and 400 mg/kg orally. Animal model of multiple sclerosis was developed in male mice 6 to 9 weeks of age were fed a diet of chow mixed with 0.2% cuprizone over the course of 28 days. The effect of CurQfen® was studied by histo pathology and change in body weight..The result of study revealed that CurQfen® 400 mg/kg exhibited significant neuroprotection and remyelination.
Keywords: curqfen®, curcumin, cuprizone, multiple sclerosis, demyelination, remyelination
CPZ, Cuprizone; MS, multiple sclerosis; CNS, central nervous system; CF, curQfen®; CRM, curcumin
Primary demyelination is a hallmark of multiple sclerosis (MS)1 and a prominent pathological feature of several other inflammatory diseases of the central nervous system (CNS) that is considered to contribute significantly to functional neurological deficits. It is a complex immune mediated process that involves the active destruction and phagocytosis of myelin by activated microglia/macrophages2 and can be accompanied by death of the Myelin-forming oligodendrocytes by apoptotic3–5 or necrotic3,6 processes. Fatigue is an early symptom in MS, often the earliest. The disease can wax and wane for up to 30 years, but in perhaps half of all cases it steadily progresses to severe disability and premature death. Most patients have a disease course consisting of inflammatory relapses accompanied by a wide range of neurological symptoms, followed by periods of remission. Over half of these patients eventually transition to a secondary progressive stage with no apparent relapses but continual neurological decline. It is believed that this progressive deterioration is due to axonal damage and loss, contributed in part by chronic demyelination. Strategies to restore lost myelin are thus considered a promising treatment approach to delay disease progression and perhaps reverse neurological symptoms.
Animals: Healthy Wistar Albino male mice were collected from the animal house of Nagarjuna pharmaceuticals thodupuzha and Animals were fed with standard diet and water ad libutum. The protocol was approved at the Institutional Animal Ethical Committee (IAEC) of DPS, RIMSR, Puthuppally with IAEC No: MGU/DPS/IAEC/2016/M PHARM–16. The animals were divided into different groups and were kept for acclimatization. They were maintained in uniform climatic conditions. Curcumin (CM) 95% and CurQfen® obtained as a gift sample from Akay Flavours and Aromatics Pvt.Ltd,Cochin, Kerala, India.
Method: Male mice at 6 to 9 weeks of age were fed a diet of chow mixed with 0.2% cuprizone over the course of 28 days.7 Curcumin and CurQfen® given orally 30 minute before the cuprizone feed.Animals were induviually caged and cuprizone feed were given to mice.
Histopathology
Paraformaldehyde fixed tissues were embedded in paraffin, and serial sections (8μm) were prepared. Sections were stained with a conventional Hematoxylin and Eosin (H and E) staining method. Digital images were taken using a ×40 objective.
In vivo screening
Body weight changes in cuprizone induced mice model
On 7th,14th,21st,28th day the weight of the animal is determined followed by cpz treatment. Body weight of mice were taken each week and noted.Cuprizone treated group showed decrease in body weight when compared to that of normal control groups.Treated group show increase in body weight when compared to the cuprizone treated group
Changes in body weight of cuprizone induced mice during each 7 days
Histopathology of cuprizone induced mice brain
A shows normal brain and B (CPZ induced model) showed brain tissue showed dense infilteration by foamy macrophages,and lymphocytic infilteration.,reactive gliosis.The cytoplasm of macrophages contained ingested myelin break down products which include dense demyelinated lesion. C showed tissue with foamy macrophages and ingested myelin break down products. D showed tissue with minimal demyelinated lesions. E showed less demyelination and less myelin break down products. F showed tissue with minimal demyelinated lesions and foamy macrophages (Figure 1).
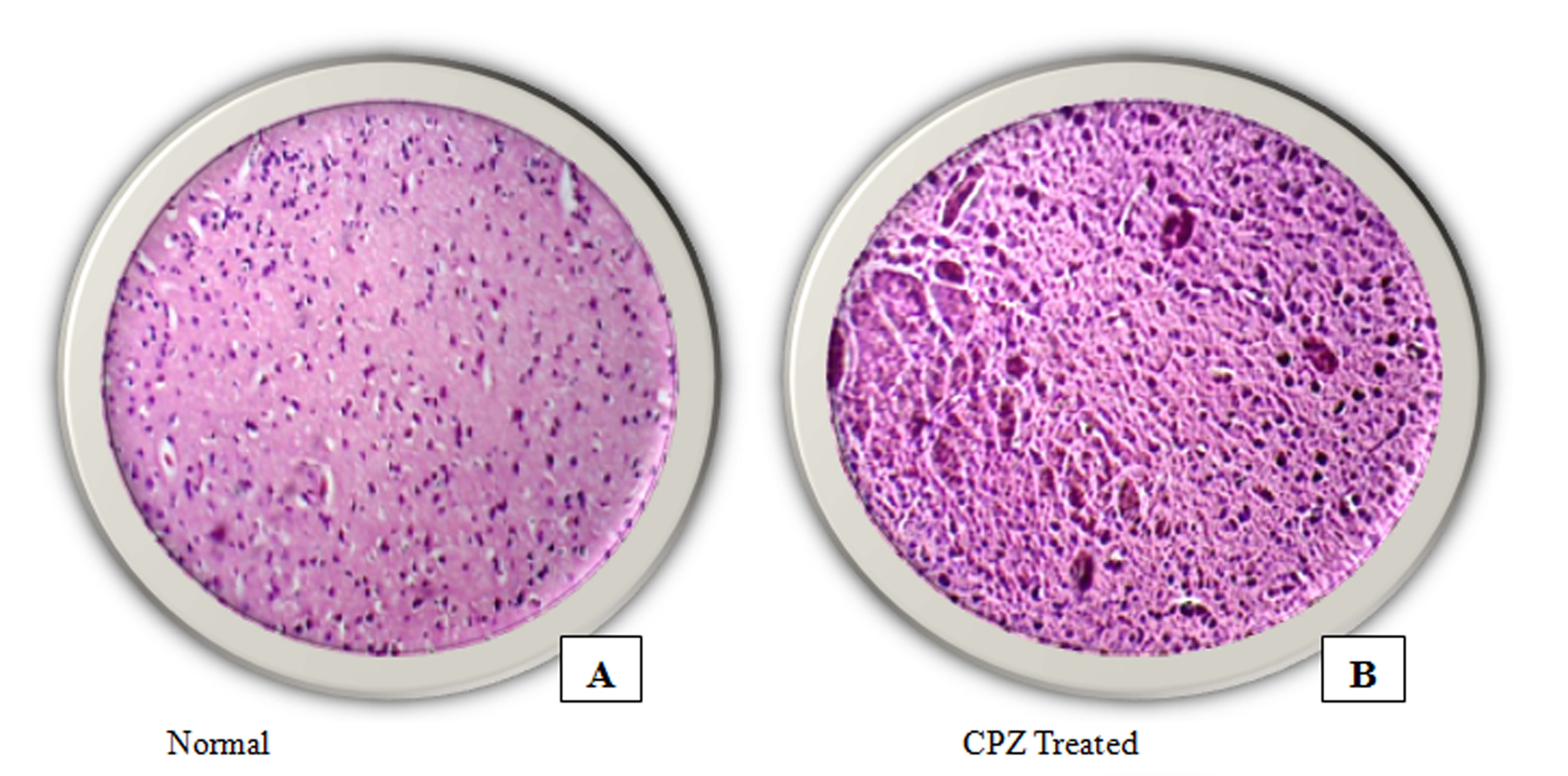
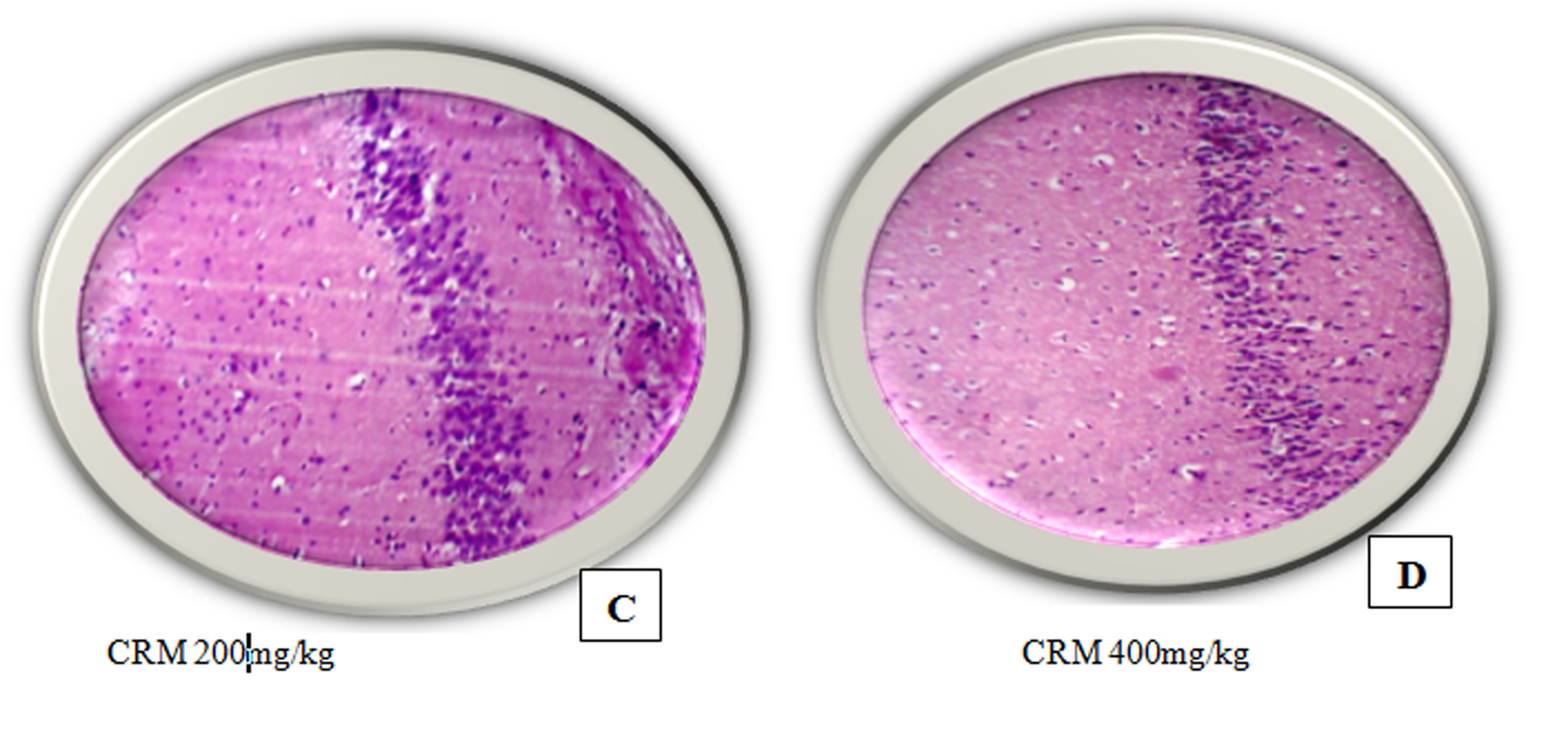
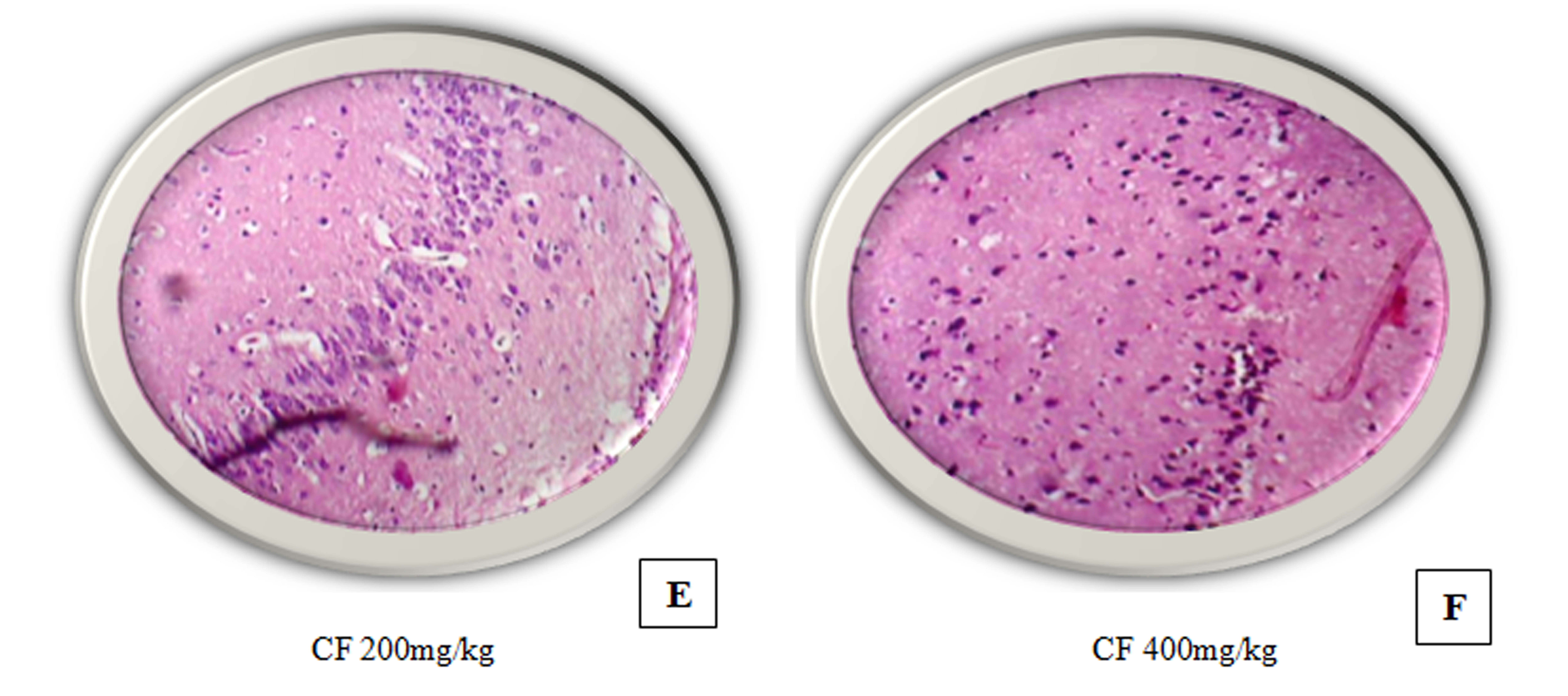
Figure 1 Histopathological analysis of brain tissue (corpous callosum) obtained from mice treated with curcumin, CurQfen® at various dosage by heamatoxylin and eosin staining.
|
Group |
Initial body Weight (g) |
7th day body Weight (g) |
14th day body Weight (g) |
21st day body Weight (g) |
28th day body Weight (g) |
|
Control (0.5ml sunflower oil) |
26.1±0.12 |
24.7±0.7 |
23.53±0.12 |
22.4±0.24 |
20.7±0.32 |
|
Toxic (CPZ treated) |
25.2±0.01 |
19.2±0.21 |
16.2±0.15 |
13.28±0.19 |
11.35±0.17 |
|
CRM200mg/kg |
25.9±0.031 |
21.44±0.15** |
20.6±0.16** |
17.21±0.19** |
16.6±0.23** |
|
CRM400 mg/kg |
26.2±0.05 |
22.12±0.14** |
21.28±0.13** |
18.98±0.16** |
18.11±0.2** |
|
CF200 mg/kg |
25.8±0.15 |
22.84±0.05** |
21.55±0.12** |
20.2±0.22** |
19.76±0.2** |
|
CF400 mg/kg |
26.0±0.24 |
23.5±0.1** |
22.55±0.12** |
21.2±0.18** |
20.13±0.2** |
Table 1 Changes in body weight of cuprizone induced mice during each 7 days.
Values are presented as mean±SEM by one way Analysis of Variance (ANOVA) followed by Dunnett-compare all vs. toxic, n=6, **p<0.01
Curcumin, a major component extracted from the rhizome Curcuma longa, has been consumed by humans as a curry spice for centuries. It has been extensively studied for its wide range of biological activities, including anti-inflammatory, anti-oxidant, anti-infection and antitumor properties, but bioavailability is a problem. The reasons for the reduced bioavailability are low intrinsic activity, poor absorption, high rate of metabolism, inactivity of metabolic products and/or rapid elimination, low plasma and tissue levels of Curcumin appear to be due to poor absorption.CurQfen®is an enhanced bioavailable form of curcumin and a unique formulation of curcuminoids from turmeric along with galactomannas from fenugreek to enhance bioavailability, and 20 times more absorption in in vivo and 15.8 time the bioavailability in human studies than standard curcuminoids, and prolonged half-life in blood plasma.8 It is clear that CPZ application to young adult mice induces demyelination in brain especially at corpus callosum.9 Although there are plenty of experiments that have been done with CPZ, it is still unclear the mechanism of CPZ induced demyelination.
According to Ventruni,10 CPZ reduces copper content of the brain in mice, and administration of copper-chelated CPZ prevented these changes in the brain. Animals treated with copper-chelated cuprizone did not show any changes. Common idea here, CPZ is a copper chelator that causes demyelination depends on copper deficiency in CNS.11,12 It has been shown that CPZ induced spongy changes in the brain of experimenting animals.13 Love noticed that CPZ causes intramyelinic edema of the cerebellar white matter, hilum of the dentate nucleus and superior cerebellar peduncle in the Wistar rats.14 The edema was associated with pyknosis but only rarely degeneration of oligodendrocytes was observed and also prominent axonal regeneration was observed despite the continued administration of CPZ. Here,histopathological results indicate that 28 days CPZ administration causes demyelination.
Body weight changes in mice were decreased when to normal control group and the treated group show increase in body weight when compare to the cuprizone treated group. Differences in mouse weight may indicate an underlying variability in the extent of white matter pathology among studies even when a 0.2% (w/w) cuprizone diet is reported. For example, a 30% reduction in weight between treated compared with non-treated mice was reported in the study that indicated development of epileptic seizures among the cuprizone-treated mice.15 In the present study, the mice lose weight during cuprizone treatment, but gain weight at the same rate as curcumin and curqfen treated mice. Cuprizone has a dose-dependent toxicity effect on mouse weight.7 Therefore variations in weight loss among studies may reflect variations in the cuprizone toxicity experienced. These differences may carry over to differences in the extent of white matter pathology and in the general health and behaviour with cuprizone treatment as well. Our histopathological results reveal that demyelinationcan be seen on corpus callosum. Histopathological reports shows that foamy macrophages and cytoplasm of the macrophages shows the ingested myelin breakdown products. And oligodendorocyte cell death occur and causes demyelination. CurQfen® 400 mg/kg treated group show less demyelinated lesions when compared to curcumin and toxic group.
It is concluded that, CurQfen® offers greater protection and remyelination against Cuprizone induced models of MS. Curqfen® had shown a promising effects in ameliorating the progression of MS in CUPRIZONE induced models. Further studies are needed to explain the mechanism of action.
None.
Authors declare that there is no conflicts of interest.

©2019 Ittiyavirah, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.