eISSN: 2377-4304


Review Article Volume 15 Issue 6
1Department of Obstetrics and Gynecology, Justus Liebig University Giessen, Germany
2Department of Surgery, St Joseph’s Hospital Balserische Foundation, Giessen
3Department of Obstetrics and Gynecology, Asklepios Hospital Lich, Germany
4Department of General Surgery and Coloproctology. Asklepios Hospital Lich, Germany
Correspondence: Dr. Gerold Link, Department of Obstetrics and Gynecology, Justus-Liebig-University Giessen Muehlenweg 16 52349 Dueren, Germany, Tel +49-2421-51488, +49-170- 4949238
Received: November 30, 2024 | Published: December 24, 2024
Citation: Link G, Meinhold-Heerlein I, Zimmer JB. Preventive strategies and surgical solutions for the treatment of prolapse disorders: new findings and old insights. Obstet Gynecol Int J. 2024;15(6):297-306. DOI: 10.15406/ogij.2024.15.00775
Background: Diseases of the female pelvic floor, ranging from genital descensus to prolapse, are benign conditions that increase with advancing age and can lead to a reduction in quality of life that requires treatment. The topic of this review concerns the historical development of preventive and therapeutic surgical options in the management of pelvic organ prolapse.
Method: Selective literature research of reviews, original papers, (German / European) guidelines and current textbooks based on personal experience and perspectives.
Results: The incidence of prolapse disorders will probably double by the year of 2050. As a result of increasing life expectancy, the treatment mandate is progressively focused on a collective of older and vulnerable patients. Modern preventive strategy concerns a better obstetric care for vaginal delivery, which is certainly the dominant risk factor for subsequent prolapse. In addition to the routine use of epidural anesthesia, the restrictive use of operative vaginal deliveries and avoidance of protracted labor, careful consideration should be given to particular situations in which primary caesarean section may be preferred as mode of birth.
Until the end of the last century, the most frequent surgical treatment performed for genital prolapse disorders was vaginal hysterectomy with colporrhaphy. In the last 30 years, surgical methods that preserve the uterus have become established. Surgical laparoscopy has enabled abdominal access into pelvic floor with diverse technical advantages. The new insight is that Level I fixation significantly enhances the success rate of surgical procedures targeting the anterior vaginal wall. Further optimization of postoperative stability has been achieved using transvaginal alloplastic implants in the anterior compartment. However, their use was later banned in most countries due to a considerable rate of complications. This fact has prompted a renewed focus on classic, well-established surgical techniques that do not involve alloplastic materials.
Uncommonly utilized procedures for anatomical reconstruction include the laparoscopic uterosacral ligament suspension and the vaginal modified Manchester procedure with amputation of the cervix. The surgical management of total prolapse in elderly, frail women with co-morbidities poses a significant challenge. In such cases, the primary focus is on resolving the functionality, while genital anatomical reconstruction becomes a secondary consideration. Occlusion of the vaginal canal through surgical procedures such as the LeFort colpocleisis or the modified Labhardt operation can offer relatively simple, low-risk solutions that are indeed very effective in cases of advanced prolapse. A great number of patients treated with these techniques are very satisfied with the procedure, mainly due to the improvement of intestinal and bladder functions. Few women express regret about undergoing this operation, as the loss of the ability to engage sexual intercourse is not a major concern in this collective.
Conclusion: Progress in the prevention and intervention of pelvic floor disorders continue to advance steadily. However, not every innovation proves successful in the long term. In this respect, the further development of surgical methods for extreme cases that rely on traditional prolapse surgery is not a mere historical oddity but an essential part of an effective treatment concept in the 21st century.
Keywords: pelvic organ prolapse, pelvic floor protection, pelvic floor surgery, laparoscopic uterosacral ligament suspension, Manchester procedure, LeFort colpokleisis, Labhardt perineoplasty
Descents of the female organs, which are supported in their physiological position by the ligaments of the pelvic floor, are common benign diseases in women that can potentially occur at any age. Depending on the degree of severity, the clinical significance of such prolapse conditions can range from an incidental finding during a gynecological examination without any symptoms to a condition that severely affects quality of life, nearing disability.
Method: Selective literature research of reviews, original papers, AWMF (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften) guidelines and current textbooks based on personal experience and perspective were used to prepare this article.
According to a large epidemiological study in the USA, a normal anatomical situation without any prolapse was found in just under a quarter (24%) of over 1000 women aged between 18 and 83 years (Pelvic Organ Prolapse Quantification [POP-Q] stage 0). POP-Q stages 1 and 2 were represented by 38% and 35% of the women examined, respectively, and only 2% of the collective were in the more advanced POP-Q stage 3.1 A total prolapse (POP-Q stage 4) was not observed.
In terms of age distribution, the peak incidence of prolapse disorders occurs in women between 70 and 79 years of age.2 In this age group, 40% of women are affected by pelvic floor dysfunction, including genital prolapse with disabling symptoms and a reduction in quality of life.3 Due to the demographic trend of an aging population, the proportion of women in the United States suffering from pelvic floor disorders is expected to increase by 50% by 2050.2 This will inevitably result in a shift of prolapse disorders to a group of patients who are more likely to suffer from diverse clinical comorbidities and frailty. This fact must be considered when the indication for surgical treatment is made
Known facts and triggering diseases in the development of genital descensus
The most important factors that can promote or cause a prolapse disorder include the ageing process, a genetic predisposition and a history of vaginal deliveries. The classification of the various potentially causal factors into a pathophysiologically oriented risk stratification is the achievement of Bump and Norton (1998),4 who divided the genesis of prolapse of the female pelvic organs into four categories. Using these categories, a successive chain of events concerning predisposing, provoking, aggravating and decompensating factors is described (Table 1).
|
Predisposing |
Genetic |
|
Race |
|
|
(→ White > African-American) |
|
|
Provocative |
Pregnancy and childbirth |
|
Surgery in the small pelvis (Hysterectomy) |
|
|
Myopathy |
|
|
Neuropathy |
|
|
Aggravating |
Obesity |
|
Smoking |
|
|
Diabetes mellitus |
|
|
Lung diseases (COPD) |
|
|
Occupation (heavy lifting) |
|
|
Constipation |
|
|
Decompensating |
Ageing |
|
Menopause |
|
|
Poor general condition |
|
|
Pharmacotherapy (glucocorticosteroids) |
Table 1 Potential risk factors for prolapse disorders (according to Bump and Norton, 1998)
Effect of hormone deficiency on the pelvic floor after the menopause
The significance of the menopause for the pathogenesis of pelvic floor disorders is the subject of controversial debate.5 However, it is well established that a decrease in systemic estrogen levels, resulting in an estrogen-depleted environment in the pelvic organs, contributes to change in the composition and strength of collagen, which can reduce the support function of the fascial apparatus.6 Lower serum estrogen concentrations and reduced expression of estrogen receptors in the pelvic floor ligaments of postmenopausal women with prolapse, compared to those without prolapse indicate the importance of the hormonal environment.7 Therapeutically, this is reflected in long-term local estrogen treatment, which can lead to a significant remission of symptoms in cases with moderate prolapse. In this context, menopause is not merely a consequence of aging, but an independent risk factor, regardless of age or parity.6
The disorganization of the visceral pelvic architecture, resulting from the various risk factors, is primarily caused by a weakening of the collagen in the connective tissue of the endopelvic fascia and ligaments. According to Petros' integral theory, the normal position of the pelvic organs can no longer be maintained.8 Any injuries to the muscular pelvic floor, previously compensated for by an intact supporting apparatus, can become symptomatic and lead to visible anatomical deviation. The concept of different levels of support, introduced by DeLancey, enabled precise localization of the weak points primarily affected by the prolapse process and laid the foundation for differentiated surgical approaches.5,9
Recent findings on pelvic floor protection during vaginal birth
The most significant event in pelvic floor disorders concerns parity. The likelihood of suffering prolapse or stress incontinence increases steadily with each vaginal birth, starting after the first birth with a 4-fold elevated risk of descensus and a doubled risk of stress incontinence. These potential consequences of natural childbirth have produced an increase in elective caesarean section as a perceived solution to protect the pelvic floor. This shift is particularly notable given the significant reduction in morbidity and complications associated with primary caesarean section over the last decade, mainly due to the widespread use of spinal anesthesia and the adoption of Misgav-Ladach technique.10 Therefore, the maternal and obstetrical risks associated with elective caesarean delivery is currently only slightly higher than the general health risks of spontaneous vaginal delivery.
The previous discussion on the risk of damage to the pelvic floor caused by vaginal birth primarily focused on the size of the newborn and the potential for injury during forceps-assisted delivery, as well as the possible damage to the neuromuscular and muscular pelvic structures, including partial or complete tearing of the levator ani muscles. Another risk factor is a prolonged expulsion period.11 These and other peculiarities of the birthing process, along with the consideration of obstetric complications in the medical history - such as higher-grade perineal tears with or without fecal incontinence and/or pre-existing urinary incontinence - have attracted attention of obstetricians and midwives under the concept of “pelvic floor protective obstetrics”. This approach aims to counterbalance the decision for elective caesarean section in cases where operative delivery offers no clear benefit. This is particularly relevant for women with a low-risk profile, which likely applies to the majority of pregnant women.12
However, even without the special focus on the pros and cons of caesarean section, many publications over the last decade considered the options of prophylaxis of pelvic floor damage.13 DeLancey et al. recently specified clinically plausible measures that primarily concern the risk reduction of injuries to the levator ani muscles in an expert review.14
These include (Table 2):
|
Situation of women |
Risk-reducing provision |
Effect for reduced risk |
|
Pregnancy |
Young age at first pregnancy (< 30 - 25 years) |
Better quality of muscular pelvic structures |
|
Normal BMI (≤ 25 kg/m2) |
Smaller levator opening area |
|
|
Low parity |
Limited risk of pelvic floor disorders |
|
|
Pelvic floor exercises |
Reduction of urinary incontinence but no effect on levator ani avulsion |
|
|
|
Early induction of labor (39 weeks of gestation) |
Smaller infant → less stretch on the pelvic floor tissues |
|
Vaginal delivery First stage of labor |
Reduction of anxiety and stress |
Reduction of muscular tension |
|
Routine offers of epidural anesthesia |
Relaxation of the pelvic floor |
|
|
|
Prevention of protracted birthing process |
Less mechanical stretch on the pelvic floor |
|
Vaginal delivery Second stage of labor |
Fetal occipitoanterior presentation |
Smaller presenting fetal head diameter |
|
Slow gradual but not prolonged expulsive stage of birth |
Improved viscoelastic tissue relaxation |
|
|
Warm perineal compresses |
Favorable change in tissue properties due to heat application |
|
|
Minimizing vaginal operative delivery |
Prevention of unphysiological forces on the pelvic floor |
|
|
|
Preference vacuum rather than forceps |
Reduction of traction force and peak pressure |
|
Significant Increased risk for birth related injuries |
Elective (primary) caesarean section |
Complete prevention of stretch forces on the pelvic floor in women with a high-risk profile |
Table 2 Prophylaxis of pelvic floor damage during pregnancy and childbirth12–14
No protective effect for levator ani injuries or urinary incontinence was detectable for:
Furthermore, pelvic floor protection includes the prevention of protracted birthing process, including the first stage of labor, and minimizing vaginal operative delivery as far as possible. On the other hand, the observation that a muscle in a state of contraction is more prone to injury than one in a relaxed state can be therapeutically significant for a group of patients at increased risk for pelvic floor disorders. This is particularly relevant with the widespread use of epidural anesthesia during childbirth, which facilitates muscle relaxation and can potentially reduce the risk of damage.14 Thus, by using a differentiated obstetric risk assessment model, elective caesarean section should be only reserved for patients with a high-risk profile.12
Medical efforts to bring prolapse of the female genital tract under control date back to the end of the 16th century - at that time using pessaries made of metal, wood, wax or cork. The possibility of introducing surgical corrections instead of the previous foreign bodies only became possible in the 19th century following the discovery of asepsis by Lister (1827 - 1912) and anesthesia by Morton (1818 - 1868).15
The first surgical methods focused on closure of the vagina (LeFort, 1877) and the interposition of the uterus (Watkins and Wertheim, 1899). However, with the growing anatomical knowledge of the supporting structures of the pelvic floor, associated with names such as Mackenrodt, Fothergill and Halban, reconstruction techniques were developed early on with the aim of tightening weakened fascia and reattaching torn ligaments. Even the sophisticated apical fixation of the uterus or the prolapsed vaginal stump was already reported by the end of the 19th century.16
Paradigm shift from hysterectomy to operations with preservation of the uterus
Although these findings were taken up by Amreich and Richter in the second half of the 20th century and developed into a standardized technique,17 surgical treatment of descensus and prolapse took a different path and for over 100 years stereotypically favored vaginal hysterectomy with anterior and posterior colporrhaphy as the standard treatment for any form of prolapse.
The primary reason for the exceptional popularity of this surgical principle was the experience that any prolapse could be effectively corrected by removing the uterus. Even in cases of total prolapse, the vaginal stump could be completely restored after high peritonealization and fixation of the sacrouterine and round ligaments at the vaginal base.18
Additionally, the common mindset of the time played a role, with the widespread belief in society that the uterus was dispensable after the end of the reproductive period. Thus, removal of the uterus, if not advisable, at least was considered to be protective and certainly not nonsensical, because hysterectomy can eliminate a possible focus of disease with potentially malignant dimensions in an organ that is not deemed necessary.
This view has undergone a significant change in the last 20-30 years, which is reflected internationally in a continuous decline in hysterectomy numbers.19 Modern diagnostic methods for the early detection of (pre)malignant cervical abnormalities (endo-/ectocervical cytology smear, HPV screening, differential colposcopy with biopsy) have contributed to this development, as well as the further development of organ-preserving alternatives to hysterectomy in the treatment of bleeding disorders and other benign diseases such as endometrial ablation, surgical hysteroscopy, selective embolization of the uterine fibroids and laparoscopic myomectomy.20 The progress in medical technology was accompanied by a general new subjective appreciation of the uterus by women, even after the end of its reproductive function.
The shift in operative paradigm from removal of the uterus to organ-preserving techniques has been incorporated in the surgical arsenal for treating prolapse disorders, especially in cases where no genuine pathology of the uterus but only a change in its position is present. The observation that the uterus plays a central position in the small pelvis, much like the keystone in a cathedral vault, and that hysterectomy creates a weak point in the pelvic floor architecture – thereby potentially promoting a subsequent descensus, such as an enterocele - has provided a further scientific argument in favor of preserving the uterus. An overview presenting current interventions for the treatment of pelvic organ prolapse is given in Table 3.
|
Route |
Intervention |
Characteristics |
Area of indication |
|
Vaginal Procedures |
Hysterectomy with colporrhaphy |
Standard treatment for over 100 years |
Additional pathology of the uterus |
|
a) Anterior colporrhaphy with autologous tissue |
Standard technique for central cystocele |
Primary repair in the anterior compartment |
|
|
b) Anterior colporrhaphy with alloplastic mesh |
Superior to a) using autologous tissue but significant complication potential |
G, A, S: very large or recurrent cystocele, special demands on stability. |
|
|
Prohibited in AS |
|||
|
Apical suspension of the cervix or the vaginal cuff |
Improving results of a) and b) by 10 -15% |
Today concomitant procedure with a) and b) |
|
|
Modified Manchester procedure (MM) |
Partially preserving uterus, apical support + anterior colporrhaphy. |
POP-Q stages II, III, preferably in post-menopausal women |
|
|
Physiological direction of vaginal axis |
|||
|
Sacrospinous hysteropexy |
Preserving uterus, apical support + anterior colporrhaphy. |
All POP-Q stages. Competing procedure with MM. Inferior to MM regrading combined outcome |
|
|
|
Risk of cystocele recurrence. Dorsal deviation of vaginal axis |
||
|
Laparoscopic (L) and Robotic (R) Procedures, optionally in combination with vaginal surgery |
L-Sacrocolpopexy, |
Apical support using alloplastic material |
All POP-Q stages: Predominant apical prolapse with moderate cysto-/rectocele |
|
L-Pectopexy, |
|||
|
L-Bilateral suspension according to Dubuisson |
|||
|
L-Uterosacral ligament suspension (L-USLS) |
Apical support using nonabsorbable polypropylene sutures. |
||
|
Physiological direction of vaginal axis. No mesh |
|||
|
R-unilateral pectineal suspension (rUPS) according to Brucker |
Apical support using nonabsorbable ethibond suture. No mesh |
||
|
Vaginal Occlusion Procedures, optionally in combination with other procedures |
LeFort colpocleisis |
Closure of the genital hiatus. Loss of ability on sexual intercourse |
Symmetrical complete genital prolapse with large bulging masses |
|
Modification of Labhardt's high perineoplasty |
Cases not suitable for the LeFort technique due to prolapse dissymmetry |
Table 3 Current surgical interventions for the treatment of pelvic floor disorders
G, Germany; A, Austria; S, Switzerland; AS, Anglo-Saxon countries
Some randomized studies have shown that there is no significant difference between the results of primary treatment concerning removing or preserving of the uterus, so that total hysterectomy is now only part of a descensus operation if there is a corresponding additional indication.21,22 However, to reliably exclude uterine pathology, it is advisable to perform a curettage before uterus-preserving surgery or a supracervical hysterectomy (LASH) or at least an office endometrial biopsy.
Newer surgical treatment options applying surgical laparoscopy and using alloplastic implants
While the vaginal approach dominated for a very long time in descensus operations, the progressive development of laparoscopic procedures and robotic surgery in the last decade enabled the application of the abdominal route, which was previously only used in exceptional cases for organ´s position correcting procedures due to the need for an invasive laparotomy. The minimally invasive techniques have rapidly become popular resulting in widely used mesh-assisted fixation techniques such as sacrocolpopexy, pectopexy or bilateral suspension according to Dubuisson. If the uterus is still present, a preceding supracervical hysterectomy (LASH) with subsequent fixation of the cervical stump is currently widely used. In contrast, a very interesting new variant is the robotic unilateral pectineal suspension (R-UPS) according to Brucker, which requires only a non-absorbable ethibond suture without a mesh interposition for the correction of the descensus. In a group of 33 patients who underwent robotic surgery, the average operating time for isolated R-UPS was only 46.5 minutes, with 15 minutes for the console time (docking of the robotic unit).23 Optionally, R-UPS can be combined with other interventions to correct the prolapse.
A patient´s fundamental condition for the application of the minimally invasive approach is a sufficient cardiopulmonary state of health allowing extreme Trendelenburg positioning, sometimes over a longer time. However, this requirement is not always met in all patients. For this reason, particularly in very elderly women laparoscopic surgery is seldom an option.
Regarding the question of whether a prolapse disorder requires surgical treatment at all, the presence of subjective complaints is of protruding importance. As descensus, regardless of its severity, does not generally pose a health risk, but rather addresses the comfort of body sensation, there is usually no need for conservative, much less surgical treatment, if the patient does not complain of prolapse symptoms such as feeling of a bulge, downward pressure, foreign body sensation or bladder or bowel emptying disorders.
If symptoms were present, the focus continuing this triage was exclusively on the compartment to which the symptoms were attributable. The translation to the most common bladder descensus in the sense of a central cystocele previously implied, that the advice on surgical treatment was restricted to only perform an anterior colporrhaphy. Any concomitant moderate descensus of the uterus or vaginal stump was not included in this treatment concept. However, subsequent studies have shown that the risk of recurrence after anterior colporrhaphy decreases significantly if the middle compartment is surgically stabilized ideally at the same time.24,25
Conventional cystocele surgery with autologous tissue repair is a standard procedure and very frequently performed. It is still considered a good option for a primary situation according to the German Descensus Guidelines, despite its elevated recurrence rate by around 46% when performed isolated. These results, according to more recent evidence, can be significantly improved by adding a simultaneous apical support (reduction in recurrence rate to 31%).26
The vaginal correction of a cystocele using alloplastic mesh is clearly superior to reconstruction with autologous tissue. The basal success rate is 83% compared to 54% without mesh. If the principle of concomitant apical fixation is also implemented, the success of the anterior repair can be increased to 93% compared to 69% when using the patient's own tissue.21 However, the notorious advantages of better objective and subjective outcomes are counterbalanced by the significant complication potential with transvaginal meshes, including pain, erosion, dyspareunia, bleeding, vaginal discharge. These complications often necessitate additional interventions and have led to numerous medico-legal disputes, particularly in Anglo-Saxon countries, ultimately resulting in the banning of alloplastic materials. This outcome was largely influenced by the previously uncritical use of meshes that were initially too small-pored and too heavy, combined with occasionally insufficient surgical expertise.
Continuous advancements have significantly enhanced the biological properties of today's exclusively monofilament polypropylene meshes, particularly in terms of pore size (100–150 μm) and weight (< 25 g/m²). These biomechanical improvements have reduced the complication rate from 12% - associated with now-banned implants - to approximately 2%. However, despite being less common, alloplastic materials inserted laparoscopically for abdominal apical suspension are inherently subject to the same potential complications as transvaginally implanted meshes. Unlike in Anglo-Saxon countries, transvaginal mesh implants for the correction of cystocele still be used in Germany, Austria, and Switzerland under specific conditions outlined in the German-Austrian-Swiss guidelines. These implants can be applied by a urogynecologically specialized surgeon after thoroughly explaining all treatment options to the patient, provided one of the following situations is present:
Other European countries have adopted more restrictive regulations for the approval and use of alloplastic materials. For instance, in the Netherlands, transvaginal implants are only allowed in certified centers, while in France, their use has been banned except within the context of clinical trials.27 These examples highlight the ongoing debate within Europe regarding the appropriate use of transvaginally inserted meshes. As a result, no universally accepted surgical algorithms can currently be recommended.
Turning away from mesh implants and renaissance of long-established surgical methods
For this reason, surgical methods that dispense with the use of meshes but still implement the principle of apical suspension are still relevant, not least because of their undisputed international acceptance.
A particular challenge arises in elderly patients with comorbidities, where time-consuming, complex surgeries or endoscopic interventions requiring Trendelenburg positioning are often unsuitable due to their compromised general medical condition. In countries where the use of alloplastic prostheses is not prohibited, prolapse in older women is typically addressed with transvaginal mesh. However, as most countries worldwide currently restrict or ban mesh surgery, a therapeutic dilemma has emerged. This situation has led to an international resurgence in occluding colpocleisis procedures, which trace their origins to the earliest days of surgical prolapse treatment.
The following section outlines four less common surgical techniques for treating prolapse. These include two reconstructive procedures that utilize the uterosacral ligaments, without the use of alloplastic materials, and two occluding colpocleisis procedures (Table 4).
|
Intervention |
Surgical principle |
Surgical route |
Vaginal function |
(Mean) operative time (Range, SD) |
(Mean) Reoperation rate (Range) % |
Scale of satisfaction |
(Mean) Follow-up time (Range) months |
|
Uterosacral Ligament Suspension (USLS) Patients of all ages |
Shortening of both uterosacral ligaments |
Laparoscopic |
Completely preserved |
Ronsini et al.29 |
2,5 (1,9 – 2,9) |
NR |
NR (3 – 24) |
|
N=267 |
|||||||
|
121 (60 – 190) |
|||||||
|
Favero et al.30 |
0 |
Very satisfied |
20,3 (11 – 27) |
||||
|
N = 6 |
|||||||
|
36 (30 – 42) |
|||||||
|
Modified Manchester Procedure (MM) Patients of all ages |
Plication of the uterosacral and the cardinal ligaments, amputation of the uterine cervix |
Vaginal |
Completely preserved |
Enklaar et al.31 |
In operated compartment: 0 |
Very much better and much better: 82,1% |
24 |
|
N=434 |
|||||||
|
62 (48 – 80) |
|||||||
|
LeFort colpocleisis Elderly patients |
Inversion of the vagina by front-toback sewing of denuded vaginal areas and fibromuscular layer |
Vaginal |
Considerably restricted |
Zebede et al.33 |
1,3 |
Cured or or greatly improved: 92,9% |
≥ 3 |
|
N=310 |
|||||||
|
NR |
|||||||
|
Grzybowska et al.34 |
NR |
Very much better and much better: 88 – 100% |
NR (1 – 60) |
||||
|
49 studies |
|||||||
|
150 ± 23 |
|||||||
|
Modified Labhardt perineoplasty Elderly patients |
Incorporation of wound edges resulting from routinely anterior colporrhaphy into high perineoplasty |
Vaginal |
Completely abolished |
Van Huisseling36 |
Due to recurrence: 0 |
N = 25 |
NR (6 – 28) |
|
N=30 30 |
Very much better and much better: 92% |
||||||
|
|
Table 4 Characteristics of less known operations without mesh material for the treatment of pelvic organ prolapse
NR, not reported
Laparoscopic uterosacral ligament suspension (L-USLS) involves a modern alternative to the widely used vaginal uterosacral fixation (V-USLS) of the apex.28 The anatomical advantage of the uterosacral ligaments depends on the fact that stabilizing the pelvic floor the physiological axis of the vagina remains preserved and does not result in a deviation, which is frequently observed after sacrospinous fixation, for example. Although a recent meta-analysis showed no significant difference between L-USLS and V-USLS in terms of recurrence, reoperation, and complication rates,29 the laparoscopic approach still had a distinct advantage, consisting of better visualization of vulnerable structures in the pelvis, which greatly increases the safety of the procedure. In addition to the rectum, blood vessels, and autonomic nerves, particularly injuries to the ureters - which represent the most common complication of vaginal uterosacral ligament suspension (V-USLS) - can largely be avoided with careful surgical technique.29
L-USLS can be used to suspend the cervix thus preserving the uterus or to promote fixation of the vaginal stump after hysterectomy. If indicated, it may also be combined with vaginal colporrhaphy. Concerning the technical procedure, L-USLS is initiated with exploration of the retroperitoneum and direct identification of both ureters. These structures are gently moved laterally to permit proper access to the medial pararectal space and the visualization of the uterosacral ligaments (USLs). At this point, permanent running sutures (Polypropylene 0) are placed along the USLs and the peritoneum of the cul-de-sac, optionally, if a larger enterocele is present. Both lines of sutures get anchored in the posterior wall of the cervix or the vagina, respectively, and peritoneum.30
Regarding the mean operation time, the literature shows considerable fluctuations reaching from an additional 36 minutes for the pure L-USLS procedure without the creation of ports in a small group of 6 patients who primarily underwent oncological surgery, to 120 minutes including the placement of ports in a systematic review involving 267 patients.29,30 Due to the advantages mentioned above, the low complication rate, especially concerning the ureter, and the fact that no alloplastic material is needed, L-USLS has become an increasingly popular method in the treatment of descensus disorders.
Internationally, the transvaginal approach remains the most frequently employed route for the surgical correction of pelvic organ prolapse. The Modified Manchester procedure (MM), a vaginal technique derived from earlier methods, has gained renewed attention in Europe over the past 10–15 years, particularly in the Netherlands.
Among uterine-preserving procedures, the modified Manchester procedure competes with sacrospinous hysteropexy, which slightly tends to be preferred for the primary treatment of uterine prolapse. Both techniques are typically performed in combination with an anterior colporrhaphy. Nevertheless, a recent randomized study comparing the Manchester operation with sacrospinous hysteropexy showed that sacrospinous hysteropexy (global success rate 77.0%) was inferior to the Manchester operation (global success rate 87.3%) when evaluated using a combined outcome measure that included the absence of prolapse associated symptoms:
The surgical technique involves the following steps: a circumcision of the vaginal epithelium around the cervix is made. The vaginal epithelium is mobilized, and the bladder is dissected from the cervix. The uterosacral ligament complex is mobilized. Extraperitoneal plication of the uterosacral ligaments at the posterior side of the uterus using four absorbable sutures is performed. The most cranial suture is placed through the vaginal wall of the posterior fornix. The cardinal ligaments are plicated on the anterior side of the cervix. An anterior colporrhaphy is performed, as mostly is indicated. The cervix is then amputated distal to the attachment of the cardinal and uterosacral ligaments. The sutures through the uterosacral ligaments are tied, except the most cranial suture which ends in the posterior fornix. The cervix is reconstructed by means of Sturmdorf sutures, thus covering the amputated cervix with vaginal mucosa. Subsequently, the most cranial suture through the uterosacral ligament is tied whereby the cervix gets elevated.
One possible adverse effect of the Manchester procedure is stenosis of the cervical canal after amputation of the portio. For this reason, special attention must be paid to ensure that the ostium of the neocervix is not covered by the Sturmdorf sutures, as otherwise postoperative dysmenorrhea may occur in premenopausal patients. The procedure is naturally contraindicated if a future pregnancy cannot be definitively excluded.
In comparison with laparoscopic uterosacral ligament suspension, it is noteworthy that in the Manchester procedure the same structure of the uterine supporting apparatus is used for the correction of the descensus. The only difference, apart from the different access routes, concerns the mechanism of elevation, which is performed laparoscopically by isolated shortening and vaginally by median conjunction of both uterosacral ligaments. Thus, the physiological direction of the vaginal axis is preserved in both operations. The large number of studies on the Manchester procedure reflects the growing interest in the rediscovery and further development of the method inaugurated over a century ago by surgeons in England.31
Old insights for the treatment of massive genital prolapse in old and frail female patients
In principle, every POP-Q stage can be corrected by laparoscopic uterosacral ligament suspension and the modified Manchester procedure. However, neither one of these techniques, nor laparoscopic fixations to the promontory or pectineal ligament, can adequately control a complete genital prolapse with large bulging masses.
Furthermore, elderly female patients over the age of 80 have a 13.6 times higher risk of mortality after urogynecological procedures compared to women in a younger age group.32 The causes of increasing surgical morbidity are mainly complications related to anesthesia and the long duration of surgery. For this reason, advanced vaginal prolapse in elderly patients requires alternative procedures to the time-consuming reconstructive methods, which are characterized by surgical simplicity, reliable anatomical control, low demands on anesthesia (spinal anesthesia or local anesthesia with sedation) and reduced operating time.33 These requirements are met by vaginal occlusion techniques. If additional pelvic floor disorders are present, such as urinary incontinence, rectal or anal prolapse, the prolapse operation can be combined with other necessary procedures, such as suburethral sling, transanal surgery according to Longo or even laparoscopic rectopexy provided that the patient's state of health allows Trendelenburg positioning.
However, since colpocleisis operations at least partially occlude the vagina, their use is limited to patients who express no desire to engage sexual intercourse. Figure 1 shows an example of the pre- and postoperative site of the external genitalia in an over 80-year-old, still spry female patient. The urogynecological problem in this case consisted of a combination of total uterovaginal prolapse and rectal prolapse. An interdisciplinary procedure was performed with the surgical department, correcting the gynecological prolapse by LeFort colpocleisis and the rectal prolapse by laparoscopic rectopexy.
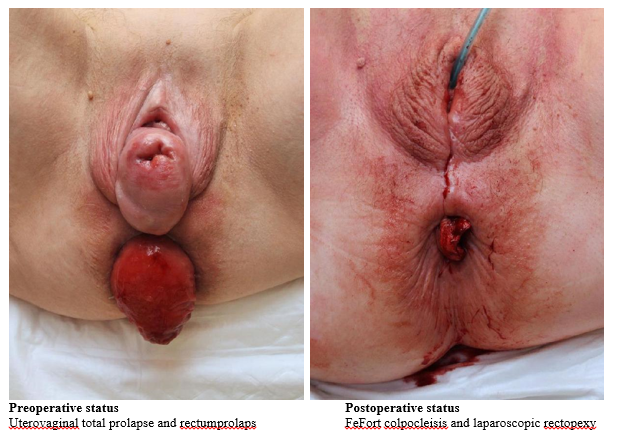
Figure 1 Pre- and postoperative site of the external genitalia in an over 80-year-old female patient suffering from a combination of total uterovaginal prolapse and rectal prolapse. Using an interdisciplinary procedure the gynecological prolapse was corrected by LeFort colpocleisis and the rectal prolapse by laparoscopic rectopexy.
Currently, the most used method is the LeFort colpocleisis. First described in 1877, it has proven to be a valuable surgical option for older women with complete prolapse of the uterus or vaginal stump. At the onset of the procedure, rectangular portions of the anterior and posterior vaginal wall are demarcated with a sterile marker and vaginal epithelium is dissected off the underlying fibromuscular layers anteriorly and posteriorly, leaving epithelial strips on the sides to create channels. The denuded areas are then sewn together front-to-back in progressive rows of 2-0 vicryl interrupted sutures. Additionally, the underlying fibromuscular tissue is gathered by sutures thus creating a supportive layer and avoiding cavities. After the vagina has been inverted, the superior and the inferior margins of the vagina are sutured horizontally. Subsequently, a high perineorrhaphy is performed. Concomitant incontinence interventions may be associated if indicated.33,34
In a collective of 310 elderly women (81.3 ± 5.3 years), 288 patients (93%) reported a cure or significant improvement of prolapse symptoms during a follow-up period between 2 and 292 weeks. The anatomical success rate achieved 98%. Of the 6 patients (2%) who suffered from a recurrence, 4 underwent re-colpocleisis. The functional outcome showed a significant improvement in the category of urinary symptoms concerning stress and urge incontinence, micturition and urinary retention, and in the category of bowel symptoms a significant decrease in constipation, obstructed defecation and fecal incontinence.33
Recently, the favorable outcome for patients after LeFort colpocleisis was confirmed in a comprehensive compilation of 49 studies.34 The reported operation times were not insubstantial because of the heterogeneous procedures due to indicated additional interventions (Table 4), but altogether time for occlusive prolapse surgery was shorter compared to reconstructive procedures and enabled earlier discharge from the hospital.
Many studies report that the lost ability of cohabitation in patients treated with colpocleisis is not a reason for regretting their decision. Rather, the assessment of the procedure is dominated by the fact that the expectations dedicated to the operation were fulfilled regarding the management of the prolapse. Provided that the perineorrhaphy was carried out not too extensively, the LeFort technique often preserves a short vaginal access or a small swale though not sufficient for normal penetration. In other studies, the rate of patients who regret the procedure varied between 1.2 to 12.9%. A good half of the patients remained sexually active even after colpocleisis using clitoral stimulation.35
Due to its high success rate and low complication rate (mostly limited to urinary tract infections), LeFort colpocleisis is currently a very effective and relevant technique in the arsenal of pelvic floor surgical therapies. Potentially, it may be considered a first-line treatment for older women with advanced pelvic organ prolapse.33
The optimal application of the LeFort colpocleisis technique requires that the procidentia symmetrically involves the anterior and posterior vaginal wall with the cervix or the apex in between, so that mirror-image rectangles can be deepithelialized and subsequently inverted. On the other hand, a frequently existing genital prolapse, characterized by asymmetrical dominance of only one compartment, can easier be corrected by means of an operation using exclusively a raised perineoplasty, thus achieving the obliterative effect. In the original technique of Labhardt (1932), such a high perineum was created by surgically fusing the lateral parts of the introitus and the labia minora, thereby closing the vagina subtotally.
With the aim to counteract any risk of recurrence due to dilatation of the small vaginal opening remaining with this method, a Modification of Labhardt's high perineoplasty was developed for the treatment of massive pelvic organ prolapse in very old patients (36). The surgical principle intends to occlude this opening by using left open wound edges of an always carried out anterior colporrhaphy and stitching them into the high perineoplasty. In brief detail, an upper and a lower U-shaped incision of the vaginal introitus running parallel are made, each starting laterally at the introitus of the vagina, approximately 1,5 cm under the level of the urethral meatus. The introital incisions enclose a wide tissue wedge which will be denudated. Subsequently, the most proximal point of the anterior wound edge resulting from preceded anterior colporrhaphy is connected by a first suture to the corresponding point of the posterior vaginal wall in the midline of the upper incision. A second suture is placed laterally 1 cm above the first suture taking the following sequence and course: from posterior wall to one of the anterior vaginal flaps, continuing to the other anterior vaginal flap and back through the posterior vaginal wall on the other side. The following sutures correspond to the described second suture thus step by step closing the inner wound edges together with the anterior vaginal wall until the vagina is closed till the point under the urethral meatus. Following the tissue wedge is reefed working one´s way up to the urethral meatus thus building a high perineoplasty. Closing the skin ends the procedure. The duration of the operation averages 30 minutes.
Thirty patients with severe prolapse aged between 70 and 91 years were treated following the described method. No additional interventions were performed. One recurrence was noted without initiating further surgery. The few complications of the procedure concerned one case each of hemorrhage and wound dehiscence and four cases of urinary retention. Subjectively, the surgical results were predominantly assessed as very good. In particular, the feeling of well-being was highly improved. None of the patients regretted the operation. The frequency of urinary incontinence decreased postoperatively, while the number of continent patients doubled. For fecal incontinence, there was no difference between the preoperative and postoperative situation.36
Based on the encouraging results in a still limited collective, the conclusion may be drawn that the modification of the Labhardt colpocleisis offers an elegant and minimally invasive alternative with potential to adequately treat advanced prolapses. The special benefit of this technique lies in its application to cases that are not suitable for the LeFort technique due to the anatomical characteristics of the prolapse.
None.
None.
The authors declare that they have no competing interests.

©2024 Link, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.