eISSN: 2377-4304


Research Article Volume 16 Issue 4
1Jefe de Unidad Ginecología y Mastología Hospital Juan A. Fernández, Buenos Aires, Argentina
10Jefe de Departamento Quirúrgico Hospital Juan A. Fernández, Buenos Aires, Argentina
2Residente de Mastología. Hospital Juan A. Fernández, Buenos Aires, Argentina
3Médico de planta de Ginecología y Mastología Hospital Juan A. Fernández.Buenos Aires, Argentina
4Residente de Tocoginecología Hospital Juan A. Fernández, Buenos Aires, Argentina
5Jefe de Sección de Mastología Hospital Juan A. Fernández, Buenos Aires, Argentina
6Jefe de Sección Oncología Hospital Juan A. Fernández, Buenos Aires, Argentina
7Médico de planta de Anatomía Patológica Hospital Juan A. Fernández, Buenos Aires, Argentina
8Servicio de Diagnóstico por imágenes Hospital Juan A. Fernández, Buenos Aires, Argentina
9Jefe de División Ginecología y Mastología Hospital Juan A. Fernández, Buenos Aires, Argentina
Correspondence: Giselle M Pizarro, División Ginecología y Mastología, Jefa de Unidad de Ginecología, Hospital Juan A. Fernández, Ciudad Autónoma de Buenos Aires, Argentina
Received: July 20, 2025 | Published: July 31, 2025
Citation: Pizarro, G, Casado M, Barchuk S, et al. Evaluation of quality of care indicators in a Mastology Unit: comparative analysis of the last decade. Obstet Gynecol Int J. 2025;16(4):133-143. DOI: 10.15406/ogij.2025.16.00800
Introduction: A multidisciplinary approach to breast cancer (BC) is essential through the creation of Mastology Units (MU). To assess their effectiveness, there are indicators of quality of care developed by Scientific Societies, the most widely used being the one proposed by the European Society of Mastology (EUSOMA).
Objectives: To evaluate the quality of care in the UM of the Fernandez Hospital through the analysis of quality of care indicators (EUSOMA). To compare the results of indicators in two periods: pre- and post-pandemic. To identify which reached the minimum standards and ideal values.
Materials and methods: Retrospective descriptive study analyzing data from patients with CM treated between 2015-2019 and 2021-2024. Population characteristics and percentage of patients who met the indicators were recorded comparing both periods.
Results: We evaluated 462 patients with CM treated at our institution: 284 (2015-2019) and 178 (2021-2024). The minimum standard value was achieved in the earlier period in 72% of indicators (18/25) vs 74% (17/23) in the later period and the ideal in 25% vs. 48% (11/23), respectively. The minimum standards were reached in: clinical-imaging preoperative diagnosis, anatomopathology and multidisciplinary evaluation, surpassing the first period. The delay at the beginning of treatment remained below the standard, without significant changes. In surgical management, the standards were met, improving with respect to the previous period, with a higher proportion of breast reconstructions. In terms of radiant treatment, the standards were maintained without significant changes. As for systemic treatment, accessibility to hormonal therapy and adjuvant and neoadjuvant chemotherapy treatment was achieved, showing improvement. In the follow-up, the minimum standard was reached without significant changes.
Conclusion: We highlight the relevance of defining indicators with values adjusted to the characteristics of our country in order to identify strategies to achieve a better quality of care.
Keywords: mastology, breast cancer, magnetic resonance imaging, chemotherapy
BC, breast cancer; EUSOMA, European Society of Mastology; MRI, magnetic resonance imaging
In Argentina, breast cancer is the most frequent oncological disease among women and the one that causes the most deaths, with more than 21,500 new cases diagnosed and more than 6,300 deaths per year.1 To achieve control of this pathology in the medium term, efforts must focus on its early detection and the implementation of relevant treatments.
Faced with this reality, Mastology Units have emerged as key organizational models for the comprehensive approach to breast pathologies, promoting the convergence of different specialties in a collaborative environment.2 It represents a functional unit and the most appropriate way of working to diagnose, treat and follow up patients with breast cancer, since it allows the optimization of available resources, the setting of common objectives and the execution of coordinated actions, with the purpose of reducing the number of suboptimal treatments, the complications inherent to them and guaranteeing quality standards in the care provided.3–5
Worldwide, particularly in Europe and the United States, programs aimed at quality control and continuous improvement have been established in centers dedicated to Mastology. Their objectives are to determine whether the proposed actions are carried out as planned, with good coverage of the population and whether they meet the needs of the users.1,6
In countries such as Argentina, quality of care in Mastology is a priority issue, given its direct impact on clinical outcomes and patient experience. Therefore, it is essential to analyze how these units are implemented in the local context, identify the challenges they face and evaluate strategies aimed at quality control and continuous improvement.
The worldwide relevance of monitoring the performance of Breast Centers through a set of Quality Indicators is demonstrated by the various initiatives carried out at international and national level. Although the Argentine Society of Mastology has defined fundamental and complementary components for a service to be considered a Mastology Unit, quality of care indicators have not yet been defined at the national level, which would be essential to follow up patients over time in a standardized manner and to easily recognize when attention is required to improve specific areas in the provision of health services.3,6
It is for this reason that for the analysis of this work we will use the revised indicators proposed by the European Society of Mastology (EUSOMA),7 corresponding to the 2017 update,8 to make a comparison and analyze the degree of compliance with them by the Mastology Unit of the Juan A. Fernández Hospital. As a precedent we have the presentation by Pizarro et al.9 from our institution, published in 2020, based on the same parameters.
Objectives
A retrospective descriptive study was performed by analyzing data collected from medical records and the database of the Breast Pathology Section of the Hospital General de Agudos Juan A. Fernández and the Breast Cancer Registry of the Argentine Society of Mastology.
The records of patients diagnosed with stages 0 to III breast cancer who were treated in the service in two periods were evaluated: the first between January 2015 and December 2019, already published by our institution9 and between January 2021 and December 2024. Patients seen during 2020 due to the COVID-19 pandemic and those with stage IV onset will be excluded from the study, given that there are substantial differences with respect to therapeutic management and treatment goals.
To describe the characteristics of the population, we recorded age, stage at diagnosis (according to the TNM classification of the American Joint Committee on Cancer - AJCC - eighth edition),10 type of primary treatment performed (surgery or neoadjuvant chemotherapy), tumor size, degree of axillary involvement, histological and immunohistochemical type, delay time at the start of treatment, adjuvant treatment and follow-up.
For the analysis of quality of care, the indicators proposed and described by EUSOMA in its latest update in 2017 were used (Appendix 1). Of the 25 process indicators, two (u and v) referring to systemic treatment were excluded as they do not apply in the current period (2021-2024) due to the modification of the neoadjuvant treatment standards for Her2neu + and triple-negative breast cancer.11–15 For each of them, the proportion of patients who complied with their respective percentage and their relationship with the minimum acceptable standard value, with the ideal value and the difference between the real value and the minimum acceptable value were described. The results were compared between the two periods.
We evaluated 462 breast cancer patients treated at our institution: 284 patients in the period 2015-2019 and 178 patients in the period 2021-2024(Appendix 2)(Table 1).
Table 1 describes the characteristics of the populations in both periods.
|
|
2015-2019 |
2021-2024 |
||
|
n |
% |
n |
% |
|
|
Total patients |
284 |
178 |
||
|
Ca invasive primary surgery |
199 |
70 |
94 |
53 |
|
Ca invasive QTN1 |
57 |
20 |
70 |
39 |
|
Ca in situ |
28 |
10 |
14 |
8 |
|
Median |
Range |
Median |
Range |
|
|
Age (years) |
58 |
24-95 |
57 |
31-99 |
|
Initial Stage2 |
n |
% |
n |
% |
|
0 |
28 |
10 |
14 |
8 |
|
1 |
101 |
36 |
50 |
28 |
|
2 |
111 |
39 |
94 |
53 |
|
3 |
44 |
15 |
20 |
11 |
|
Tumor Size3 |
n |
% |
n |
% |
|
Tis |
28 |
10 |
14 |
8 |
|
T1 |
138 |
49 |
58 |
33 |
|
T2 |
89 |
31 |
65 |
36 |
|
T3 |
15 |
5 |
39 |
22 |
|
T4 |
14 |
5 |
2 |
1 |
|
Axillary Involvement4 |
n |
% |
n |
% |
|
N05 |
173 |
61 |
126 |
78 |
|
N1 |
68 |
24 |
29 |
18 |
|
N2 |
25 |
9 |
7 |
4 |
|
N3 |
18 |
6 |
0 |
0 |
|
Histologic Type |
n |
% |
n |
% |
|
NST |
210 |
74 |
130 |
73 |
|
Lobular |
21 |
7 |
13 |
7 |
|
Ca in Situ |
28 |
10 |
14 |
8 |
|
Other |
25 |
9 |
20 |
11 |
|
Initial Immunohistochemistry6 |
n |
% |
n |
% |
|
Luminal A - Like (Re + Rp ≥20 Ki 67 |
||||
|
Low G1-2 Her Negative) |
88 |
36 |
66 |
40 |
|
Luminal B - Like (Re + Rp ≥20 Ki 67 Low G1-2 Her Negative) Luminal B - Like (Re + Rp ≤20 Ki 67 |
||||
|
High G3 Her Negative) |
82 |
33 |
60 |
37 |
|
Luminal B Her2neu + (Re + Rp ≤20 Ki |
||||
|
67 High G3 Her Positive) |
24 |
10 |
8 |
5 |
|
Her2neu Pure (Re- Rp- Her2neu +) |
15 |
6 |
9 |
6 |
|
Triple Negative (Re- Rp- Her -) |
38 |
15 |
16 |
10 |
|
No Complete Data |
9 |
- |
5 |
|
|
Total Luminal |
194 |
79 |
134 |
82 |
|
Delay to initiation of treatment (days) |
Median |
Range |
Median |
Range |
|
General (surgery + chemotherapy) |
59 |
10-198 |
86 |
7-288 |
|
Surgery |
62 |
10-198 |
115 |
14-288 |
|
Chemotherapy |
53 |
18-178 |
73 |
7-205 |
Table 1 Characteristics of the population
1.QTN=Neoadjuvant Chemotherapy. Stages according to AJCC TNM 8th edition8. 3. Tumor size. Tis: carcinoma in situ, T1: tumor ≤ 2 cm, T2: tumors between 2.1 and 5.0 cm, T3: tumor larger than 5 cm, T4: tumor with skin and/or chest wall involvement, or inflammatory carcinoma8. 4. Initial axillary involvement. N0: no axillary involvement, N1: up to 3 positive nodes, N2: between 4 and 10 positive nodes, N3: more than 10 positive nodes, or supra/infraclavicular involvement.8 5. 6. In invasive carcinoma.
In the 2015-2019 period, the minimum standard value was reached in 72% of the indicators (18/25) and the ideal value in 25% (6/24), while in the 2021-2024 period, the minimum value was exceeded in 74% (17/23) and the ideal value in 48% of the indicators (11/23) (Graph 1).
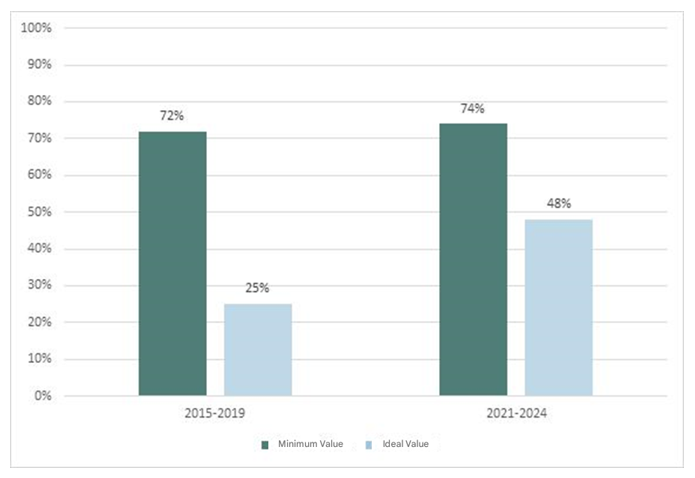
Figure 1 Graph 1 Comparison of results of compliance with quality indicators between the periods 2015-2019 and 2021-2024.
Table 2 summarizes the results of the analysis of the quality of care indicators evaluated in the 2015-2019 period, with their comparison with the minimum standards and ideal values, with the respective differences in favor or against according to each result (Appendix 3).
|
Indicator (details and definitions in materials and methods) |
Total sl: (n) |
Total patients (u) |
No data (n) |
Percentage (%) |
Minimum standard (%) |
Ideal value (%) |
Difference (Δ%) |
|
Clinical and imaging diagnosis |
|||||||
|
a. Proportion of patients with preoperative mammography + breast and axillary ultrasound |
230 |
249 |
35 |
92 |
90 |
95 |
2 |
|
Preoperative diagnosis |
|||||||
|
b. Proportion of patients with invasive carcinoma with axillary staging (ultrasound +/- biopsy)) |
225 |
250 |
34 |
90 |
85 |
95 |
5 |
|
c. Proportion of patients with carcinoma with a presurgical histological/cytological diagnosis |
220 |
249 |
35 |
88 |
85 |
90 |
3 |
|
Procnosisjpredictwe Anatomopathological characterization |
|||||||
|
d. Proportion of patients with complete record of prognostic/predictive factors in invasive carcinoma |
224 |
237 |
19 |
95 |
2 |
98 |
0 |
|
e. Proportion of patients with record of prognostic factors in carcinoma in situ |
22 |
24 |
4 |
92 |
95 |
99 |
3 |
|
Waiting time |
|||||||
|
I. Proportion of patients with a time interval to treatment start ≤6 weeks from the first consultation |
86 |
284 |
0 |
30 |
80 |
90 |
50 |
|
MRI availability |
|||||||
|
g. Proportion of cases examined with MRI (excluding neoadjuvant therapy) |
9 |
190 |
37 |
5 |
10 |
NA, |
5 |
|
h. Proportion of patients in neoadjuvant therapy who underwent MRI |
31 |
57 |
0 |
54 |
60 |
90 |
6 |
|
Multidisciplinary evaluation |
|||||||
|
i. Proportion of patients presenting to a multidisciplinary meeting |
245 |
251 |
33 |
98 |
90 |
99 |
8 |
|
Loco regional surgical treatment |
|||||||
|
j. Proportion of patents (invasive cancer) who received a single surgery for the primary tumor (excluding reconstruction) |
239 |
256 |
0 |
93 |
0 |
90 |
13 |
|
k. Proportion of patients lie situ cancer) who received a single surgery (excluding reconstruction) |
25 |
28 |
0 |
88 |
70 |
90 |
19 |
|
l. Proportion of patients who received immediate post-mastectorny breast reconstruction |
10 |
84 |
0 |
12 |
40 |
NA. |
28 |
|
Adjuvant radiotherapy |
|||||||
|
m. Proportion of patients with invasive cancer who received post-conservative surgery radiotherapy |
161 |
164 |
0 |
98 |
90 |
95 |
8 |
|
n. Proportion of patients who received post-mastectomy radiotherapy )(with indication) |
55 |
59 |
0 |
93 |
90 |
95 |
3 |
|
Avoid overtreatment |
|||||||
|
o. Proportion of patients with a negative wino who had SL NB (excluding neoadjuvant therapy) |
151 |
161. |
0 |
94 |
90 |
95 |
4 |
|
p. Proportion of patients with invasive carcinoma in whom BCG was performed and ≤5 lymph nodes were removed |
162 |
168 |
0 |
96 |
90 |
95 |
6 |
|
q. Proportion of patients with invasive carcinoma ≤ 3 cm who received conservative surgery (excludes BRCA patients) |
137 |
166 |
0 |
82 |
70 |
85 |
12 |
|
r. Proportion of patients with carcinoma in situ ≤ 2cm who received conservative surgery |
17 |
18 |
3 |
94 |
80 |
90 |
14 |
|
s. Proportion of patients with carcinoma in situ in whom axillary surgery was rot performed |
18 |
28 |
0 |
64 |
97 |
99 |
33 |
|
Systemic treatment |
|||||||
|
t. Proportion of patients with hormone-sensitive factors (invasive carcinoma) who received endocrinotherapy |
198 |
203 |
0 |
98 |
85 |
90 |
13 |
|
u. Proportion of patients with ER-negative tumors, 21 cm, and/or axilla + who received adjuvant CT |
19 |
21 |
0 |
90 |
85 |
95 |
5 |
|
v. Proportion of patients with HER2+ invasive tumors ≥ 1 cm and/or axilla + who received CT and adjuvant trastuzumab |
32 |
37 |
2 |
86 |
85 |
95 |
1 |
|
w. Proportion of patients with HER2+ invasive tumors treated with NCT who received trastuzurneb in NCT |
15 |
18 |
0 |
83 |
90 |
95 |
7 |
|
x. Proportion of patients with inflammatory or locally advanced carcinoma who received NCT |
31 |
32 |
12 |
96 |
90 |
95 |
6 |
|
Follow-up |
|||||||
|
y. Proportion of patients with routine follow-op every 6-12 months post-treatment |
281 |
284 |
3 |
99 |
95 |
99 |
4 |
Table 2 Results for the 2015-2019 period
1. Patients who meet the indicator criteria. 2. The positive percentage difference above the minimum standard indicator is shown in green, and the negative percentage difference is shown in red. 3. NA = Not Applicable. 4. MRI = Magnetic Resonance Imaging. 5. Total number of patients who apply for the indicator. 6. ER = Estrogen Receptors. 7. Trastuzumab - specific monoclonal therapy against the Her2neu cell receptor. 8. SLN = sentinel lymph node biopsy. 9. CT = chemotherapy. 10. NQT = neoadjuvant chemotherapy
Table 3 summarizes the results of the analysis of the quality of care indicators evaluated in the period 2021-2024, with their comparison with the minimum standards and ideal values, with the respective differences in favor or against according to each result.
|
Indicator (details and definitions in materials and methods) |
Total patients (u) |
No data (n) |
Percentage (%) |
Minimum standard (%) |
Ideal value (%) |
Difference (Δ%) |
|
|
Clinical and imaging diagnosis |
|||||||
|
a. Proportion of patients with preoperative mammography + breast and axillary ultrasound |
176 |
178 |
0 |
99 |
90 |
95 |
9 |
|
Preoperative diagnosis |
|||||||
|
b. Proportion of patients with invasive carcinoma with axillary staging (ultrasound +/- biopsy)) |
162 |
164 |
0 |
99 |
85 |
95 |
14 |
|
c. Proportion of patients with carcinoma with a presurgical histological/cytological diagnosis |
173 |
178 |
0 |
97 |
85 |
90 |
12 |
|
Procnosisjpredictwe Anatomopathological characterization |
|||||||
|
d. Proportion of patients with complete record of prognostic/predictive factors in invasive carcinoma |
163 |
164 |
0 |
99 |
95 |
98 |
4 |
|
e. Proportion of patients with record of prognostic factors in carcinoma in situ |
13 |
14 |
0 |
93 |
95 |
98 |
-2 |
|
Waiting time |
|||||||
|
I. Proportion of patients with a time interval to treatment start ≤6 weeks from the first consultation |
51 |
178 |
0 |
29 |
80 |
90 |
-51 |
|
MRI availability |
|||||||
|
g. Proportion of cases examined with MRI (excluding neoadjuvant therapy) |
42 |
100 |
7 |
42 |
10 |
Not applicable |
32 |
|
h. Proportion of patients in neoadjuvant therapy who underwent MRI |
59 |
70 |
1 |
84 |
60 |
90 |
24 |
|
Multidisciplinary evaluation |
|||||||
|
i. Proportion of patients presenting to a multidisciplinary meeting |
162 |
176 |
2 |
92 |
90 |
99 |
2 |
|
Loco regional surgical treatment |
|||||||
|
j. Proportion of patents (invasive cancer) who received a single surgery for the primary tumor (excluding reconstruction) |
160 |
164 |
0 |
98 |
80 |
90 |
18 |
|
k. Proportion of patients lie situ cancer) who received a single surgery (excluding reconstruction) |
13 |
14 |
0 |
93 |
70 |
90 |
23 |
|
l. Proportion of patients who received immediate post-mastectorny breast reconstruction |
18 |
67 |
0 |
27 |
40 |
Not applicable |
-13 |
|
Adjuvant radiotherapy |
|||||||
|
m. Proportion of patients with invasive cancer who received post-conservative surgery radiotherapy |
87 |
91 |
5 |
96 |
90 |
95 |
6 |
|
n. Proportion of patients who received post-mastectomy radiotherapy )(with indication) |
15 |
17 |
0 |
88 |
90 |
95 |
-2 |
|
Avoid overtreatment |
|||||||
|
o. Proportion of patients with a negative wino who had SL NB (excluding neoadjuvant therapy) |
|
68 |
9 |
97 |
90 |
95 |
7 |
|
p. Proportion of patients with invasive carcinoma in whom BCG was performed and ≤5 lymph nodes were removed |
100 |
113 |
3 |
88 |
90 |
95 |
-2 |
|
q. Proportion of patients with invasive carcinoma ≤ 3 cm who received conservative surgery (excludes BRCA patients) |
88 |
100 |
4 |
88 |
70 |
85 |
18 |
|
r. Proportion of patients with carcinoma in situ ≤ 2cm who received conservative surgery |
13 |
14 |
0 |
93 |
80 |
90 |
13 |
|
s. Proportion of patients with carcinoma in situ in whom axillary surgery was rot performed |
12 |
14 |
0 |
86 |
97 |
99 |
-11 |
|
Systemic treatment |
|||||||
|
t. Proportion of patients with hormone-sensitive factors (invasive carcinoma) who received endocrinotherapy |
125 |
127 |
7 |
98 |
85 |
95 |
13 |
|
u. Proportion of patients with ER-negative tumors, 21 cm, and/or axilla + who received adjuvant CT |
Not applicable |
||||||
|
v. Proportion of patients with HER2+ invasive tumors ≥ 1 cm and/or axilla + who received CT and adjuvant trastuzumab |
Not applicable |
||||||
|
w. Proportion of patients with HER2+ invasive tumors treated with NCT who received trastuzurneb in NCT |
16 |
17 |
0 |
94 |
90 |
95 |
4 |
|
x. Proportion of patients with inflammatory or locally advanced carcinoma who received NCT |
48 |
52 |
0 |
92 |
90 |
95 |
2 |
|
Follow-up |
|||||||
|
y. Proportion of patients with routine follow-op every 6-12 months post-treatment |
171 |
176 |
2 |
97 |
95 |
99 |
2 |
Table 3 Results for the 2021-2024 period
1. Patients who meet the indicator criteria. 2. The positive percentage difference above the minimum standard indicator is shown in green, and the negative percentage difference is shown in red. 3. NA = Not Applicable. 4. MRI = Magnetic Resonance Imaging. 5. Total number of patients who apply for the indicator. 6. ER = Estrogen Receptors. 7. Trastuzumab - specific monoclonal therapy against the Her2neu cell receptor. 8. SLN = sentinel lymph node biopsy. 9. CT = chemotherapy. 10. NQT = neoadjuvant chemotherapy
Table 4 compares the results of the quality indicators in the periods 2015-2019 and 2021-2024.
|
Indicator (details and definitions in materials and methods) |
Result (%) period |
Result (%) period |
Difference between results (Δ%) of the |
p |
|
Clinical and imaging diagnosis |
||||
|
a. Proportion of patients with preoperative mammography + breast and axillary ultrasound |
92 |
99 |
7 |
0.002 |
|
Preoperative diagnosis |
||||
|
b. Proportion of patients with invasive carcinoma with axillary staging (ultrasound +/- biopsy)) |
90 |
99 |
9 |
0.0004 |
|
c. Proportion of patients with carcinoma with a presurgical histological/cytological diagnosis |
88 |
97 |
9 |
0.0008 |
|
Procnosisjpredictwe Anatomopathological characterization |
||||
|
d. Proportion of patients with complete record of prognostic/predictive factors in invasive carcinoma |
95 |
99 |
4 |
0.009 |
|
e. Proportion of patients with record of prognostic factors in carcinoma in situ |
92 |
93 |
1 |
0.08 |
|
Waiting time |
||||
|
I. Proportion of patients with a time interval to treatment start ≤6 weeks from the first consultation |
30 |
29 |
-1 |
0.15 |
|
MRI availability |
||||
|
g. Proportion of cases examined with MRI (excluding neoadjuvant therapy) |
5 |
42 |
37 |
<0.0001 |
|
h. Proportion of patients in neoadjuvant therapy who underwent MRI |
54 |
84 |
30 |
0.0002 |
|
Multidisciplinary evaluation |
||||
|
i. Proportion of patients presenting to a multidisciplinary meeting |
98 |
92 |
-6 |
0.007 |
|
Loco regional surgical treatment |
||||
|
j. Proportion of patents (invasive cancer) who received a single surgery for the primary tumor (excluding reconstruction) |
93 |
98 |
5 |
0.06 |
|
k. Proportion of patients lie situ cancer) who received a single surgery (excluding reconstruction) |
89 |
93 |
4 |
0.07 |
|
l. Proportion of patients who received immediate post-mastectorny breast reconstruction |
12 |
27 |
15 |
0.02 |
|
Adjuvant radiotherapy |
||||
|
m. Proportion of patients with invasive cancer who received post-conservative surgery radiotherapy |
98 |
96 |
-2 |
1 |
|
n. Proportion of patients who received post-mastectomy radiotherapy )(with indication) |
93 |
88 |
-5 |
0.08 |
|
Avoid overtreatment |
||||
|
o. Proportion of patients with a negative wino who had SL NB (excluding neoadjuvant therapy) |
94 |
97 |
3 |
0.31 |
|
p. Proportion of patients with invasive carcinoma in whom BCG was performed and ≤5 lymph nodes were removed |
96 |
88 |
-8 |
12. |
|
q. Proportion of patients with invasive carcinoma ≤ 3 cm who received conservative surgery (excludes BRCA patients) |
82 |
88 |
6 |
0.2 |
|
r. Proportion of patients with carcinoma in situ ≤ 2cm who received conservative surgery |
94 |
93 |
-1 |
0.16 |
|
s. Proportion of patients with carcinoma in situ in whom axillary surgery was rot performed |
64 |
86 |
22 |
0.061 |
|
Systemic treatment |
||||
|
t. Proportion of patients with hormone-sensitive factors (invasive carcinoma) who received endocrinotherapy |
98 |
98 |
0 |
Not applicable |
|
u. Proportion of patients with ER-negative tumors, 21 cm, and/or axilla + who received adjuvant CT |
Not applicable |
|||
|
v. Proportion of patients with HER2+ invasive tumors ≥ 1 cm and/or axilla + who received CT and adjuvant trastuzumab |
Not applicable |
|||
|
w. Proportion of patients with HER2+ invasive tumors treated with NCT who received trastuzurneb in NCT |
83 |
94 |
11 |
0.1 |
|
x. Proportion of patients with inflammatory or locally advanced carcinoma who received NCT |
96 |
92. |
-4 |
0.3 |
|
Follow-up |
||||
|
y. Proportion of patients with routine follow-op every 6-12 months post-treatment |
99 |
97 |
-2 |
0.6 |
Table 4 Comparison of the quality indicators between the periods 2015-2019 and 2021-2024
1. Patients who meet the indicator criteria. 2. The positive percentage difference above the minimum standard indicator is shown in green, and the negative percentage difference is shown in red. 3. NA = Not Applicable. 4. MRI = Magnetic Resonance Imaging. 5. Total number of patients who apply for the indicator. 6. ER = Estrogen Receptors. 7. Trastuzumab - specific monoclonal therapy against the Her2neu cell receptor. 8. SLN = sentinel lymph node biopsy. 9. CT = chemotherapy. 10. NQT = neoadjuvant chemotherapy
The analysis of quality indicators in the care of breast cancer patients is a fundamental tool for assessing the effectiveness and efficiency of the care process. Evaluating these parameters not only makes it possible to identify achievements and areas for improvement, but also to design specific institutional strategies aimed at optimizing the comprehensive approach.6 The existence of an updated and reliable institutional database is an essential condition for carrying out this type of comparative studies, and for sustaining over time a quality monitoring system that is dynamic, reproducible and useful in clinical practice.
In this study, 23 indicators proposed by the European Society of Breast Cancer Specialists (EUSOMA) in its latest update (2017) were used, which have been widely validated in European mastology units.8
Firstly, in relation to clinical and imaging diagnosis and preoperative evaluation, the data presented show high compliance and exceeding the established quality standards in terms of evaluation with mammography and preoperative axillary breast ultrasound, the proportion of patients with invasive carcinoma with axillary staging, as well as the proportion of patients with preoperative histological/cytological diagnosis. Significant improvement was achieved with respect to the 2015-2019 period in all three indicators.
In terms of prognostic/predictive anatomopathologic characterization, the quality standard was also met and exceeded in invasive carcinoma, having improved with respect to the previous period evaluated. These results coincide with those reported in certified European centers,8 where the early availability of this information has a direct impact on the therapeutic choice and on the possibility of discussing cases in multidisciplinary athenaeums with complete data.
In the case of carcinoma in situ, we have a small sample of cases (n=14) and although in only one case the information on hormone receptor expression is not available, the compliance with the complete registry of prognostic factors was 93% (13 of 14), without reaching the minimum EUSOMA standard of 95%. The case in which there was no information on hormone receptors came to our mastology unit with the anatomopathologic diagnosis made in another institution, without being able to recover the curettes for a new anatomopathologic characterization. This factor is a determining factor for the therapeutic decision (for example in adjuvant hormone therapy). However, there were no significant differences with the previous period.
Concerning the use of magnetic resonance imaging (MRI), its use was analyzed both in patients without neoadjuvant treatment and in those who received it. In the first group, it was observed that 42% of patients were evaluated with preoperative MRI, which represents a marked improvement compared to the 2015-2019 period, where only 5% was reached. This result far exceeded the minimum standard established by EUSOMA (10%), and although this indicator does not have a defined ideal value, the increase evidences a greater availability of this tool and its progressive incorporation into the diagnostic algorithm. Several studies have shown that the use of MRI in certain subgroups -such as young patients, with multifocal disease, dense breasts or a genetic background- contributes to better surgical planning and a reduction in the number of reoperations.16
In patients who underwent primary systemic treatment (neoadjuvant chemotherapy), the indication for MRI was even higher, reaching 84% of cases. This figure improved significantly with respect to the previous period (30 percentage points) and far exceeded the established minimum value (60%). MRI in the neoadjuvant setting is especially useful for assessing tumor response, lesion extension and planning surgery16. The improvement over the previous period may be related to a greater availability of hospital shifts.
One of the most critical points observed in this evaluation was the indicator of time from diagnosis to treatment initiation, which was only met in 29% of cases. Although no significant differences were found compared to the previous period (30%), this value is still well below the minimum standard of 80%. This problem is related to multiple causes and is influenced by various factors: on the one hand, after the COVID-19 pandemic, patients were slow to resume medical consultations, delaying the time from the initial consultation. On the other hand, a centralized appointment scheduling system was implemented after the pandemic, which may have initially made it difficult to access appointments in the various specialties that make up the MU, both to complete the diagnosis of CM, its staging, and the oncological evaluation. In addition, difficulties inherent to the public system persist in terms of latency in accessing oncology medications and surgical appointments. This represents an institutional constraint extrinsic to the MU, which may prevent improvements in treatment times throughout the periods evaluated. The literature supports the importance of reducing this interval: studies have shown that delays of more than 60 days until primary surgery may be associated with worse overall and specific survival for CM.17 This is also evident in the delay in starting neoadjuvant treatment, where a delay of more than 61 days from diagnosis was associated with an increased risk of death, greatly worsening the prognosis of the disease. Therefore, this is undoubtedly a critical issue to be addressed with higher-level institutional and health strategies to shorten treatment delays.18 A widely used and proven successful strategy is the incorporation of health navigators. A systematic review published in 2021 demonstrated the effectiveness of this new role in the healthcare system, reporting that patients experienced a statistically significant reduction in the time between diagnosis and treatment.19 Within the Mastology Unit, we will propose a possible circuit for patients for future improvements, as the presence of navigators could optimize access to improve these indicators.
With respect to the presentation of cases in multidisciplinary meetings, 92% compliance was achieved in 2021-2024, reaching the minimum standard value without significant changes compared to the previous period. Although the percentage decreased compared to the previous period, it was not a significant difference. We can attribute this to the fact that in the first post-pandemic period (2021) multidisciplinary articulation was difficult due to the restrictions implemented, which later recovered their usual activity. This reflects a clear consolidation of interdisciplinary work in the unit, where therapeutic decisions are discussed periodically with the active participation of mastologists, oncologists, pathologists and imaging specialists. This model, recommended internationally, improves the quality of treatment, reduces the variability of decisions and promotes safer and more patient-centered care.6,20 On the other hand, we also comply with national standards: the Argentine Society of Mastology indicates that 90% of the cases treated in the Mastology Units must be presented.4
With regard to the surgical approach to the primary tumor, very favorable results were obtained in terms of locoregional treatment indicators and those related to avoiding overtreatment. In 98% of cases of invasive carcinoma, surgical treatment was completed with a single procedure, without the need for reoperations to widen margins or complete axillary treatment. This figure far exceeds both the minimum standard (80%) and the ideal standard (90%) of EUSOMA, and shows improvement compared to 2015–2019. We observed the same in patients diagnosed with invasive carcinoma and a tumor ≤ 3 cm who were treated with conservative surgery (88%), far exceeding both the minimum standard (70%) and the ideal value (85%) with improvement also compared to the first period (82%). In the cohort of patients with carcinoma in situ, 93% of conservative surgery was achieved when the tumor measured ≤ 2 cm, also exceeding the minimum standard (80%) and the ideal value (90%), with no significant changes compared to the previous period (94%). The proportion of patients diagnosed with carcinoma in situ who underwent a single surgery was 93%, exceeding the minimum value of 70% proposed by EUSOMA, as well as the ideal value (90%). This indicator was higher than in the 2015–2019 period (89%). This positive evolution can be attributed to more accurate surgical planning in line with current treatment standards, probably linked to better preoperative characterization by imaging (especially with MRI) and correct histological diagnosis prior to surgery with intensification in the previous period.21 The decrease in surgical reoperations is directly associated with a better patient experience, lower morbidity, and optimization of institutional resources.21 On the other hand, reoperations generate higher costs for the healthcare system with a greater risk of potential complications.22
In relation to immediate postmastectomy breast reconstruction, although we experienced a considerable improvement between both periods (12% vs 27%), these results continue to be below the minimum quality standard (40%). This demonstrates the progress in interdisciplinary management with greater integration and participation of the plastic surgery service. The factors that could explain the failure to reach the standard in both periods are probably related to the limited availability of resources in the public system: the impossibility of patients to afford prosthetic material for reconstruction and the delays in obtaining it through prepaid or social security. In addition, it is important to consider that many patients in our population are elderly or have major comorbidities that may contraindicate immediate reconstruction. Despite the low rate observed, it represents a concrete improvement, especially if we consider that immediate reconstruction improves quality of life and body image, without significantly increasing complications when it is well indicated.23,24
With regard to axillary surgical management, the proportion of patients with clinically negative axillae who underwent sentinel lymph node biopsy (SLNB) was evaluated, excluding those who received neoadjuvant treatment. The result was 97%, which comfortably exceeds both the minimum standard (90%) and the ideal standard (95%) proposed by EUSOMA. This data is consistent with that of the previous period (94%) and reflects a well-established practice in the unit. We observed that the minimum standard was not achieved in the management of the axilla in carcinoma in situ, probably due to the low number of patients in the sample, but there was a significant improvement compared to the previous period (86% vs. 64%), reinforcing the previous statement. Performing BGC instead of axillary lymphadenectomy in these cases avoids surgical overtreatment, reduces associated morbidity (lymphedema, pain, functional limitation), and is widely supported by international evidence as the standard of care in patients with clinically negative axillae.25,26 In relation to the number of lymph nodes resected, we observed that the indicator is just below the minimum standard proposed by EUSOMA (88% vs. 90%), with an 8% decrease in the 2015–2019 period. This can be explained by the fact that in the second period, a greater number of patients underwent post-neoadjuvant BGC (10 vs. 30 in the first and second periods, respectively), where the average number of lymph nodes removed is higher than in primary surgery. This EUSOMA indicator does not distinguish between primary and neoadjuvant surgery, which may lead to a less accurate assessment. It would be important to continuously update quality indicators in line with treatment updates based on evidence.
In the literature, neoadjuvant treatment is recognized as the main factor in the resection of a greater number of lymph nodes. Other factors include young patients, larger tumor size, high-grade tumors, and low RE levels.27 Although the clinical impact may be low, maintaining this number within optimal parameters is important to avoid overtreatment and reduce the risk of lymphedema.28
With regard to adjuvant radiotherapy treatment in patients with invasive carcinoma who underwent conservative surgery, compliance was 96% in the period 2021–2024. This result exceeds both the minimum standard (90%) and the ideal value (95%), with no significant differences from the 2015–2019 period, when compliance reached 98%. This finding indicates that referral and coordination with the radiotherapy service has been effective and that most patients received the indicated treatment in a timely manner after surgery. Breast radiotherapy after conservative surgery is a fundamental component of locoregional treatment, as it significantly reduces the local recurrence rate and improves overall survival.29
On the other hand, 15 of the 17 patients who were indicated for postmastectomy radiotherapy received it (88%), falling short of the minimum EUSOMA standard (90%). This value is also lower than that of the previous period (93%). In the 2021-2024 period, we have a reduced number of cases compared to the previous period (17 vs. 59), which makes analysis difficult. In the cases of the two patients in the last period who did not receive treatment, the cause was their refusal to undergo it. However, it is noteworthy that this aspect should be monitored closely, given that post-mastectomy radiotherapy with indication reduces locoregional recurrences and improves disease control,30,31 especially in high-risk patients, and although only two patients did not receive it as indicated, they represent a significant percentage of this population.
As for the use of hormone therapy in patients with hormone-sensitive tumors, 98% of patients expressing hormone receptors received endocrine therapy, exceeding the minimum standard (85%) and the ideal standard (95%). This value remained stable compared to the first period (98%). This result reflects an adequate anatomopathological characterization of the tumor immunohistochemical profile, as observed in indicator d., as well as effective access to tamoxifen or aromatase inhibitors in postmenopausal patients. It also suggests that the clinical team has incorporated the use of hormone therapy as an integral part of adjuvant treatment, in line with the recommendations of all international guidelines for luminal tumors.15,32 In relation to the proportion of patients with invasive tumors expressing Her2neu who were treated with neoadjuvant chemotherapy and received trastuzumab, we observed that the indicator was met in 94% of cases, exceeding the minimum standard (90%) and improving compared to the previous period (83%). This improvement could be due to greater indication and better access to molecular therapies in recent years.11 On the other hand, 92% of patients with inflammatory or locally advanced carcinoma received neoadjuvant chemotherapy, exceeding the 90% minimum standard established by EUSOMA, as in the 2015-2019 period, when the proportion was 96%.
With regard to post-treatment follow-up, the minimum standard was achieved but not the ideal standard, with no significant difference compared to the previous period. We can see that this indicator remains the same in both periods and is extremely important for the early detection of recurrences, events related to surgical and adjuvant treatment, and their proper management.14
Thanks to the interdisciplinary management in a Mastology unit, we can thus reaffirm that many of the mandatory indicators for the optimal and adequate quality management of CM in our institution are met.
It is worth noting that we have fewer patients in the current period, which could contribute to the negative difference in some results. Given the difficulties observed, it is important to point out that various aspects are specific to the public health system and the organization of the institution and are beyond the control of the Mastology Unit, thus explaining the impossibility of directly improving some quality indicators. The extrapolation of these standards to our context has limitations: in many Argentine public health centers, including highly complex units such as ours, material resources, diagnostic and therapeutic infrastructure, and the availability of specialized personnel do not always allow us to meet the ideal values proposed. These structural differences must be taken into account when interpreting the results and designing improvement interventions adapted to the local health system.
We observed compliance with the minimum standard in 72% of the indicators in the first period and 74% in the second; and we reached the ideal value in 25% of the indicators in the first period, vs 48% in the second.
We emphasize the importance of defining our own indicators with values adjusted to the characteristics of our country and maintaining a periodic evaluation of the quality of care through them, in order to identify areas for improvement and weaknesses in the systems and create strategies to achieve a better quality of care for patients.
None.
None.
The authors declares that there is no conflict of interest.

©2025 Pizarro,, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.