MOJ
eISSN: 2379-6162


Case Report Volume 13 Issue 2
1Deparment of surgery, Universidad de San Carlos de Guatemala/ Instituto Guatemalteco de Seguridad Social, Guatemala
2Deparment of surgery, Universidad de San Carlos de Guatemala/ Hospital General San Juan de Dios, Guatemala
Correspondence: Andrés Rojas, 2168381690101, Aldea Petapilla, zona 0, Condado la Pradera, Chiquimula, Chiquimula, Guatemala
Received: June 12, 2025 | Published: June 24, 2025
Citation: Rojas AE, Rodas GR. Vascular shunt placement in damage control surgery: emergency vascular management of axillar artery and vein trauma. MOJ Surg. 2025;13(2):49-51. DOI: 10.15406/mojs.2025.13.00293
The damage control involves a series of interventions that work together to effectively stop bleeding and stabilize the patient. The estimated annual incidence of civilian vascular injuries ranges from 0.9 to 2.3 per 100,000 people. There is still room for improvement, especially in small-diameter applications, such as below-knee bypass, where all prosthetic vascular grafts, including ePTFE, perform poorly compared to autologous vein. The autologous saphenous graft can be used effectively and safely for arterial and venous vascular reconstruction to treat various conditions, including atherosclerotic occlusive disease, aneurysmal disease, venous disease, and vascular trauma.
Keywords: damage control, vascular trauma autologous saphenous graft, vascular shunt
ePTFE, expanded polytetrafluoroethylene; ASPVG, autologous saphenous graft; GSV, great saphenous vein; SVC, superior vena cava
Damage control background
The concept of damage control, originating from maritime terminology, refers to the emergency actions taken to prevent a ship from sinking during a crisis. These actions prioritize keeping the ship afloat rather than immediately completing comprehensive repairs. When applied to trauma care, damage control involves a series of interventions that work together to effectively stop bleeding and stabilize the patient. This approach focuses on preventing immediate death rather than addressing all injuries definitively at the initial stage.1
Vascular anatomy
The total length of the axillary artery, from origin to end, was 144.37 mm. The initial caliber measured 11.24 mm and narrowed to 5.65 mm after the origin of the common arterial trunk. The final portion of the axillary artery measured 4.87 mm. The second portion of the axillary artery, 62.64 mm from its origin, gave rise to the common arterial trunk. The common arterial trunk had a total length of 23.72 mm and a thickness of 6.1 mm. The caliber of the branches originating from the common arterial trunk were as follows: subscapular artery, 5.1 mm; anterior humeral circumflex, 1.66 mm; posterior humeral circumflex, 3.18 mm; and deep brachial artery, 3.73 mm.
The axillary artery is divided into three portions. The first portion is located anteriorly to the axillary vein and posteriorly to the three fascicles of the brachial plexus. The second portion is anterior to the pectoralis minor muscle, posterior to the medial fasciculus of the brachial plexus and the medial root of the median nerve, lateral to the common arterial trunk, and medial to the axillary vein. The third portion is located anteriorly to the suspensory ligament of the axilla, posteriorly to the ulnar nerve, laterally to the median nerve, and medially to the axillary vein. The organization of the brachial plexus terminal branches was altered due to the vascular variation discovered. The median nerve's medial root was positioned between the axillary artery and the common arterial trunk.
The subscapular artery ran medially, branching into the thoracodorsal and circumflex scapular arteries. The posterior and anterior humeral circumflex arteries arose independently, coursing laterally toward the surgical neck of the humerus. The posterior humeral circumflex artery continued through the quadrangular space beside the axillary nerve. Finally, the deep brachial artery joined the radial nerve, traveling distally through the triangular interval toward the posterior compartment of the arm.2
Vascular trauma
Vascular trauma accounts for roughly 3% of all trauma cases globally, based on emergency room admissions. However, major peripheral vascular trauma, defined as injuries to blood vessels larger than 4mm, comprises only 3% of all trauma injuries and 0.67% of total patients. The estimated annual incidence of civilian vascular injuries ranges from 0.9 to 2.3 per 100,000 people.3
A 34-year-old male patient, involved in a motorcycle accident under the influence of alcohol, presented to the emergency department with a heart rate of 108 bpm, respiratory rate of 22 breaths per minute, oxygen saturation of 89%, and blood pressure of 100/60 mmHg. Upon physical examination, a complex wound with active hemorrhage was observed in the left axillary region.
A dressing with hydrogen peroxide was applied to manage the hemorrhage, and the patient was subsequently transferred to the operating room. Intraoperatively, upon reopening the wound, active bleeding was confirmed. Hemostasis was achieved utilizing Kelly clamps. An examination of disrupted vascular structures revealed a 6 to 7 cm disruption of the axillary artery and vein.
The patient exhibited hemodynamic instability, requiring the placement of a central venous catheter, the administration of vasoactive amines, and a blood transfusion. Damage control surgery was initiated, involving the placement of a venous sheath in the axillary artery and a 13 Fr Nelaton catheter in the axillary vein, both ligated with 0 silk suture. Following restoration of circulation to the extremity, the procedure was concluded, and the patient was transferred to the intensive care unit to commence Phase 2 of damage control (Figure 1).
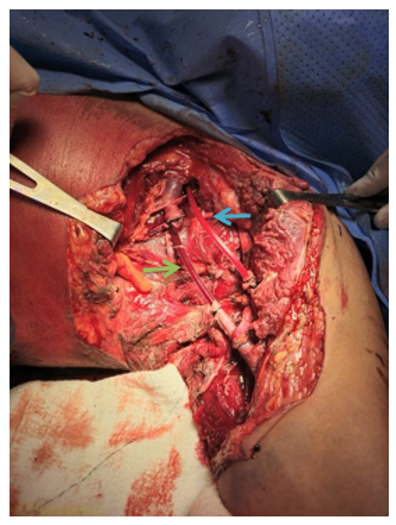
Figure 1 First phase of damage control, placement of vascular shunt. A. Green arrow, venous shunt. B. Blue arrow, arterial shunt.
The patient received intensive care management for 72 hours and was subsequently returned to the operating room, where an inverted autologous saphenous graft was successfully performed on the left axillary artery and vein (Figure 2).
Vascular Shunts
Vascular shunt placement in damage control surgery is indicated for injuries involving proximal vessels of the extremity, such as the iliac, common femoropopliteal, or superficial arteries in the lower limb, and the subclavian, axillary, and brachial arteries in the upper limb.4 The primary purpose of arterial shunting is to prevent ischemia in patients with severe vascular injury.5
In a study conducted in Ukraine, the results showed that temporary arterial bypass is useful for preventing critical ischemia and limb amputation in Level 2 patients, as in the study the patient was located at more htan30 km from the nearest hospital. This represented a long time between the moment of the incident and his arrival at the emergency service.5
The use of temporary vascular shunts is appropriate in damage control surgery, Gustilo IIIC orthopedic injuries with associated vascular injury, perfusion prior to limb replantation, complex zone III neck injuries, mass casualty scenarios, and in the transportation of a patient with vascular injury from a peripheral to a tertiary care hospital. The damaged artery is exposed, and bleeding from both ends is confirmed. A tube of similar caliber to the injured vessel is used as a temporary vascular shunt and secured with sutures on both ends.6
Indications
The following 7 situations indicate the need for a temporary vascular shunt placement:
Pros for using vascular shunts
A multicenter study conducted in the United States recently revealed that vascular shunts are being used less frequently. In nine years, only 213 such procedures were performed, equivalent to 2.7% of vascular injuries. This procedure was performed in only one case due to the surgeon's lack of surgical experience. The mortality rate, 20.4%, was due to trunk injuries, but none of these deaths were attributed to the use of vascular shunts. Finally, the limb amputation rate was 3.5%, partly due to soft tissue injuries and partly due to graft complications, but none due to the vascular shunt.8
Extremity injuries were more frequently associated with shunt use, with 65–94% of these injuries occurring, and 70–100% of these injuries were arterial compared with venous or trunk injuries.9 In a study conducted in South Africa, nasogastric tubes were used as the material used for shunt placement.10
Cons for using vascular shunts
There are certain factors that determine the placement of vascular shunts, such as: the variety of vascular traumas in terms of the location of the injury, whether they are trunk or limb type, whether they are proximal or distal, the complexity of the injury, the time of evolution, the type of referral, the center where the patient is taken, the experience of the surgeon and the resources managed in de different units.9
One might think that the areas where TVS are most commonly performed are small hospitals with limited resources and poor access to specialized surgeons; however, most are described in level 1 hospitals or trauma centers, with adequate resources and physicians capable of performing definitive repairs. However, the literature and our own experience as a level 1 trauma center support the idea that shunts are not as common in current vascular trauma cases.9
Definitive repair
The ASPVG can be used effectively and safely for arterial and venous vascular reconstruction to treat various conditions, including atherosclerotic occlusive disease, aneurysmal disease, venous disease, and vascular trauma. The graft has shown satisfactory patency and durability in the short and mid-term, with an observed patency rate of 82% at 2 years in the series published by Ketenciler et al. in 2018.
While the great saphenous vein (GSV) is commonly used in vascular reconstruction, it has limitations. Its small luminal caliber can be a major disadvantage when reconstructing main vascular branches such as the iliac, femoral, or subclavian arteries or veins. To overcome this, the GSV can be tailored into an isodiametric conduit. The spiral vein graft technique, first described in 1974 by Chiu et al. is one method used to create such a conduit and was initially used to replace the superior vena cava (SVC) in a canine model.11
Although the evidence shows that the definitive treatment can be either autologous graft with saphenous vein or expanded polytetrafluoroethylene. It is up to the surgeon to decide according to the vessel´s caliber, length and type. Both technics have advantages and disadvantages and each case and patient is different. However, for these reasons, it is important that the treating surgeon has extensive knowledge of both anatomy and existing surgical approaches. By mastering these skills, patients can receive the best option available and best prognosis despite their injuries.
To God and to all the people that made this case report possible.
The author declares there is not conflict of interest.

©2025 Rojas, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.