MOJ
eISSN: 2573-2919


Research Article Volume 9 Issue 4
1Department of Environmental Engineering, Gebze Technical University, Turkey
2Department of Environmental Engineering, Atatürk University, Turkey
Correspondence: Mehtap Dursun, Department of Environmental Engineering, Gebze Technical University, 41400, Kocaeli, Turkey
Received: July 26, 2024 | Published: August 23, 2024
Citation: Dursun M, Keskinler B, Bektaş N, et al. Preliminary treatment of landfill leachate by hydrodynamic cavitation supported by Fenton process MOJ Eco Environ Sci. 2024;9(4):178-184. DOI: 10.15406/mojes.2024.09.00324
Hydrodynamic cavitation as an effective and environmentally friendly method of treating wastewater. Massive amounts of energy may be released into the surrounding liquid during hydrodynamic cavitation, resulting in mechanical , chemical and thermal impacts. Bacteria and organic materials in sewage can be broken down by these circumstances. Furthermore, a coupling effect may be created by combining hydrodynamic cavitation with other water treatment techniques.
In this study it is aim to investigate and improve the hydrodynamic cavitation (HDC) process supported by Fenton process for the pretreatment of landfill leachate. In the second phase of the study, the effectiveness of the hydrodynamic cavitation process was investigated in conjunction with the Fenton process. The parameters such as the number of cavitation events, pH, and temperature were evaluated. The effluent was characterized and monitored for COD measurements.
The consequences of operational variables such H2O2, Fe+2 , and pH values were investigated to determine the optimal Fenton oxidation process parameters. The findings of the experiment showed that pH values were ideal for Fenton oxidation of 3.5-4.5, 30 mM H2O2, and 5 mM Fe+2.
A combined treatment process of Fe+2+H2O2, HDC + Fenton, and Cavitation alone were conducted for the treatment of landfill leachate. The results showed that the removal rates of chemical oxygen demand (COD) for the combined processes were 32.85%, 44.28%, and 7%, respectively. Temperature, pH, and the number of cavitation events were among the parameters that were assessed. The effluent was measured for COD and was characterized.
Keywords: pre-treatment, leachate, hydrodynamic cavitation, Fenton process
One of the most challenging wastewaters, landfill leachate is a highly contaminated liquid that results from the decomposing of disposed waste combined with rainwater infiltrating through the refuse layers. It has a dark color, an odor, and may contain a high concentration of both organic and inorganic contaminants.1,2 In particular, high ammonium (NH3-N) content, minimal biodegradability (BOD5/COD < 0.1), and richness in persistent organic matter are features of mature leachate.3 Leachate comes in three different varieties: young, middle, and adult. The content and biodegradability of each of these varies (Figure 1).4
Leachate from landfills is produced in large quantities and is highly contaminated, making it a potential cause of environmental contamination. High concentrations of organics, heavy metals, ammonia, and numerous other dangerous substances are present in leachate.5
Landfill leachate treatments are often divided into three main groups (Gao et al., 2015)6:
Each of of these and other methods has benefits and drawbacks of its own. Biological and physical-chemical processes combined are becoming recognized as the most efficient approach for managing and modifying highly concentrated effluents.7
Hydrodynamic cavitation is an innovative treatment process that utilizes reactive free radicals such as HO., H., HOO., and HO2. by generating regions with high temperatures and inducing liquid oxidation processes through turbulence. This method is based on circulation, where microbubbles suddenly occur and collide with the interior, leading to the formation of high temperature and pressure. The incident energy releases energy in the form of shock waves, which causes degradation of chemicals in the immediate vicinity. Therefore, HC is an effective method when coupled with the Fenton reaction, as it offers advantages such as mass transfer and reduced chemical consumption, allowing oxidation to be completed in a shorter period and reducing energy requirements for smaller reactor volumes.
The application of this treatment process has several advantages, including the use of fewer chemicals, less space requirement, and lower energy consumption (Neppiras, 1980).
The cavitation technique has been extensively studied over the last decade, and it has been successfully applied to a variety of physical, chemical, and biological processes.
This new method is shown to be more energy-efficient than many other traditional methods while still achieving the intended transformation and reducing overall processing costs. There is a great deal of promise for energy-efficient enhancement of different physical and chemical processes through cavitation.8
Cavitation has seen to be an effective option due to inclusion of extremely reactive free radicals, creating hot spots, and high turbulence resulting from the liquid circulation. An instant occurance and collapse of micro bubbles lead to high temperature and pressure. Meanwhile, an enormous amounts of energy liberates, and this energy leads to a degradation of surrounding chemicals.9 Recent major application of cavitation process is hydrodynamic cavitation (Figure 2).10
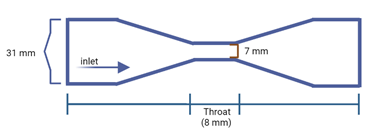
Figure 2 Fluid flow and pressure change in hydrodynamic cavitation.11
The consumption amount of chemical additives is lesser for hydrodynamic cavitation systems considering the classical oxidation systems and due the advantages of mass transfer observed in cavitation systems, reactions are ended in very short times and this leads to a smaller reactor volumes and lesser amount of energy requirements.12 Considering the ease of operation, lower requirement of chemicals, lower energy consumption, smaller footprint and the treatment achievement observed in the challenging wastes, it is thought that hydrodynamic cavitation (HDC) reactors with Fenton oxidation might be a promising treatment alternative.
Different processes have been using for the treatment of landfill leachate, e.g., chemical oxidation, coagulation/flocculation,13 adsorption,14 chemical precipitation, air stripping dissolved air/flotation and membrane filtration.15 These techniques are widely applied to landfill leachate in order to eliminate harmful and non-biodegradable substances. These pretreatment techniques are helpful before biological treatment, particularly for fresh leachate. They can also be used as a post-treatment (purification) step for partially stabilized leachate and in situations where the presence of bio-refractory components impedes the biological oxidation process. These techniques work in tandem with biological techniques to treat a particular pollutant or to increase treatment efficiency.6,7
Since the 1990s, advanced oxidation processes, or AOPs, have been widely used in the treatment of wastewater. At their basis, AOPs stem from the production of hydroxyl radicals in water. These oxidants are highly reactive and nonselective, capable of oxidizing organic molecules found in leachate. Among AOPs, the Fenton and (Fe+2/H2O2) systems are the most crucial mechanisms for producing hydroxyl radicals.16
In advanced oxidation processes, hydroxyl radicals are the main reactive intermediates responsible for the oxidation of organic matter. Hydroxyl radicals (OH •), enter quickly without selective reaction with many organic and inorganic substances in water.17 As a result, they are powerful antioxidants for both natural and synthetic organic molecules that are resistant to other processes' breakdown in natural water.18
Fenton’s reagent, a mixture of ferrous (Fe+2) ion and hydrogen peroxide (Eq. (1)), has been used widely for oxidation of organic matter in water, and to reduce the chemical oxygen demand (COD) and total organic carbon (TOC) content.19 The only change in operating the process is pH adjustment.
The use of Fe+2/H2O2 as an oxidant for wastewater treatment is attractive since iron is quite abundant and non-toxic, and a 35% hydrogen peroxide aqueous solution is easy to handle and not harmful for environment.
(1)
A specific technique that performs effective for eliminating bio-refractory materials, complex organic compounds, etc. is cavitation. The production, development, and eventual collapse of microbubbles or cavities that take place in extremely short intervals of time (milliseconds) and release significant amounts of energy across a very tiny area is known as cavitation. The ensuing consequences are very outstanding and happen simultaneously at millions of locations within the reactor.9
In a study, removal of p-nitrophenol was investigated using Hydrodynamic cavitation and Fenton process in the pilot scale plant. A combined treatment process of HC + Fenton, HC+H2O2 and HC alone was studied. For the removal efficiency lower pH values was observed more effective. It was found that although HC (alone) has low efficiency, combination of HC,Fenton process and H2O2 increases the removal efficiency. Under optimum conditions the initial concentration of 5 g / L and 10 g / L p-nitrophenol solution with a maximum removal rate was found 63.2% and 56,2%, respectively.20
In another study hybrid cavitation techniques for chemical treatment of ozone and hydrogen peroxide, hydrodynamic cavitation, acoustic cavitation, and water disinfection have been thoroughly investigated. Hybrid technique which combines hydrodynamic cavitation, acoustic cavitation, hydrogen peroxide and / or ozone has been used for reduction of bacteria, fecal coliform, total coliform and indicator microorganisms, such as fecal streptococci. It was observed that these hybrid methods are conducive than the individually applied.21
Korniluk M. et al.22 have studied the treatment possibilities of urban landfill leachate with the implementation of hydrodynamic cavitation. According to the obtained results, using hydrodynamic cavitation is not effective for the treatment of landfill leachate .However,researcher have been reported that using hybrid process such as (HC + Ozone) and(HC+H2O2) can be effective for the treatment of leachate.22
The effectiveness of various types of cavitating devices for the extension of disinfection was investigated in a study for the microbiological disifection of saltwater. It was confirmed that combining traditional water disinfection techniques like heat treatment and chlorination with hydrodynamic cavitation significantly boosted the disinfection rate overall. When 5 ppm of hypochlorite was applied as a disinfectant together with cavitation, the rate of reaction almost doubled compared to the case when only 5 ppm of hypochlorite was employed.23
Dular, M. et al.10 have investigated the use of hydrodynamic cavitation for the elimination of hazardous green microalgae (Chlorella vulgaris), cyanobacteria (Microcystis aeruginosa), bacteria (Legionella pneumophila), viruses (Rotavirus), and pharmaceuticals from water and wastewater. This study indicated that hydrodynamic cavitation is an effective choice for uses since it can be easily scaled up, long time usage, runs continuously, and has a higher removal efficiency than acoustic cavitation.10
In this study it is aim to investigate and improve the hydrodynamic cavitation (HDC) process supported by Fenton oxidation for the pretreatment of landfill leachate. In the second phase of the study, the effectiveness of the hydrodynamic cavitation process was investigated in conjunction with the Fenton process. The parameters such as the number of cavitation events, pH, and temperature were evaluated. The effluent was characterized and monitored for COD measurements.
Sanitary landfill leachate characterization
Leachate was sourced from İzaydaş (Kocaeli, Turkey) municipal solid waste landfill. It was estimated that the leachate as the mature one. amples were taken out of a buffer tank and put into plastic (HDPE) containers each time. As quickly as feasible, collected landfill leachate was kept at 4 °C. Prior to the investigations, the samples were cooled to room temperature (22°C ± 2). All tests for the environmental characterization of leachate were performed triplicate (Table 1).
|
Parameter |
Unit |
Mean Value |
|
pH |
- |
7.78 |
|
Conductivity |
ms/cm |
19,3 |
|
COD |
mg/L |
8.5 |
|
BOD5 |
mg/L |
4.195 |
|
TSS |
mg/L |
440 |
|
Total N |
mg/L |
1.33 |
|
Total P |
mg/L |
0,205 |
|
Nitrate |
mg/L |
0,216 |
|
TOC |
mg/L |
1.257 |
|
TKN |
mg/L |
176 |
Table 1 Characterization of landfill leachate sample
Analytical methods
Total Suspended Solid (TSS) measurement was performed according to the Standard Methods. Electrical Conductivity and pH measurements were conducted using Hach Sension 378 pH-conductivity dissolved oxygen meter. COD measurements were performed using open flux method and BOI measurements were performed according to the standart methods.
The experiments of combination of hydrodynamic cavitation and Fenton were applied by using various molar ratios of Fe+2: H2O2 such as 1:5, 1:10, 1:15, 1:20, 1:30, and 1:40 to determine the optimal concentration of hydrogen peroxide.
Experimental Set-up
The hydrodinamic cavitation reactor is installed on a laboratory size, with a pump with a maximum operating pressure of 5 bar and pipes connecting it to the circulation tank. The cavitation system employed the pump to generate pressure.
Cavitation system has a transparent glass tube. The experimental system was supplied with a measuring system and control valves. The entire system consisted of stainless-steel pipelines with the inside diameter of 31 mm (Figure 3).
It is operated by a Grundfos 316 stainless steel vertical shaft stainless steel pump. This pump has a capacity of 2.2 kW and has a flow capacity of 10 m3/ h. The minimum number of cavitation obtained with the 5 mm diameter orifice was 0.22. The calculation of the number of cavitation is described below.
To explain the cavitation event, the cavitation coefficient (Cv), a dimensionless number that connects the cavitation density to the flow conditions, is used.
(2)
P2= outlet pressure (mmHg)
PV= vapor pressure
V= velocity
ρ= density
To find the vapor pressure Pv of the water at the desired temperature, the following expression known as the Antonie Equation can be used.
(3)
In this equation:
A=8.07131
B=1730.63
C= 233.426 and
T, oC is denominated.
The highest flow rate of the pump through the 5 mm orifice is determined 35 L / min. Based on these data, the number of cavitation is calculated at sea level;
(4)
The leachate pretreatment of hydrodynamic cavitation was studied to improve its biodegradability. The experiments were carried out at the pressure of 5 bar.
At the begining of each experiment, the reactor was filled with 10 L of leachate. After filling the reactor, the landfill leachate pH was adjusted among 3,5-4 after adjustment of pH H2O2 and Fe+2 was added to the cavitation reactor, respectively.
Effect of pH
The pH has been observed highly important factor for effective Fenton treatment.24 The pH value influences the generation of hydroxyl radicals and thus the oxidation efficiency.25,26 The pH effect was studied among 2-10 pH values. It was determined that the lower pH values are better than the higher ones in our study. The reason for this situation is the rapid decomposition of OH. and H2O2 at hight pH values and fast reaction at low pH values.27 Figure 4 clearly shows that, as the pH increases above 3, there is a rapid increase in the COD removal rate, indicating an optimal pH 3.5-4 for the Fenton treatment process.
Lower pH values are preferred due to rapid decomposition and reaction at high pH. The graph clearly shows that the COD removal rate increases rapidly above 3 pH, suggesting a pH range of 3.5–4 ideal for the Fenton treatment process (Table 2) (Figure 4).
|
pH |
COD |
% Removal |
|
2 |
6280 mg/L |
27.45 |
|
3,5 |
5920 mg/L |
31.84 |
|
4,5 |
6150 mg/L |
29.04 |
|
5 |
6843 mg/L |
20.15 |
|
6 |
7680 mg/L |
10.38 |
Table 2 Effects of pH on Fenton process
Effect of H2O2 dosage
The concentration of H2O2 was critical in the generation of hydroxyl radicals. As a result, H2O2 (30% w/w) experiments were carried out at 15, 30, 45, and 60 mM concentrations to determine the most effective H2O2 concentration (30 mM) (Table 3) (Figure 5).
|
Fe+2 |
H2O2 |
COD(corrected)removal rate |
Residual H2O2 |
|
5 mM |
15 mM |
27,75 |
78 mg/L |
|
5 mM |
30 mM |
32,38 |
151 mg/L |
|
5 mM |
60 mM |
30,46 |
331 mg/L |
|
5 mM |
90 mM |
32,73 |
574 mg/L |
|
5 mM |
120 mM |
31,37 |
287 mg/L |
Table 3 Effect of H2O2 concentration
Effect of Fe+2 dosage
Amount of ferrous ion is one of the main parameters to influence the Fenton processes. In this study, various concentrations of Fe+2 were applied to obtain its optimal concentration.All concentration were carried out three times. The optimum Fe+2 dosage were found 5 mM.The results for Fenton are shown in Table 4 (Figure 6).
|
Fe+2 |
H2O2 |
COD removal rate |
|
3 mM |
30 mM |
6,91 |
|
5 mM |
30 mM |
31,31 |
|
7,5 mM |
30 mM |
22,34 |
|
10 mM |
30 mM |
8,30 |
Table 4 Effect of Fe+2 concentration
Fe2+/H2O2 molar ratio
In line with finding optimum Fe2+/H2O2 ratio, all the experiments conducted during this study have been shown in Table 5. Most effective Fe+2 and H2O2 concentration rate has found 5/30 mM and removal efficiency of COD has been determined %32,85.
|
Fe+2 (mM) |
H2O2 (mM) |
COD (mg/L) |
% COD Removal Rate |
H2O2/Fe+2 Molar Ratio |
|
0 |
30 |
8450 |
0,00 |
0,00 |
|
3 |
15 |
8450 |
0,00 |
5,00 |
|
3 |
27 |
8450 |
0,00 |
9,00 |
|
3 |
30 |
7960 |
3,16 |
10,00 |
|
3 |
45 |
7520 |
8,52 |
15,00 |
|
3 |
60 |
7480 |
9,00 |
20,00 |
|
5 |
15 |
5800 |
29,44 |
3,00 |
|
5 |
30 |
5520 |
32,85 |
6,00 |
|
5 |
45 |
5540 |
32,60 |
9,00 |
|
5 |
60 |
5480 |
33,33 |
12,00 |
|
6 |
30 |
6420 |
21,90 |
5,00 |
|
6 |
60 |
6020 |
26,76 |
10,00 |
|
6 |
90 |
5700 |
30,66 |
15,00 |
|
6 |
120 |
5240 |
36,25 |
20,00 |
|
6,75 |
15 |
8028 |
2,34 |
2,22 |
|
6,75 |
30 |
7812 |
4,96 |
4,44 |
|
6,75 |
45 |
7320 |
10,95 |
6,67 |
|
6,75 |
60 |
7476 |
9,05 |
8,89 |
|
8,3 |
90 |
8200 |
0,24 |
10,84 |
|
10 |
15 |
7860 |
4,38 |
1,50 |
|
10 |
30 |
7820 |
4,87 |
3,00 |
|
10 |
45 |
6900 |
16,06 |
4,50 |
|
10 |
60 |
6820 |
17,03 |
6,00 |
Table 5 Result of all conducted experiments
Effect of reaction time
The ideal operating parameters were identified, and periodic tests were carried out with different Fe+2 and H2O2 concentrations to determine the ideal reaction time (Table 6) (Figure 7) (Table 7) (Figure 8).
|
Time (Minute) |
5 mM 30 mM H2O2 |
7,5 mM 30 mM H2O2 |
10 mM 30 mM H2O2 |
|
5 |
13,4 |
24,6 |
18,8 |
|
15 |
19,8 |
19,6 |
21,8 |
|
30 |
30,6 |
26 |
29 |
|
45 |
31 |
21 |
16 |
|
60 |
1,8 |
27,8 |
23,2 |
|
90 |
9,4 |
12 |
24,6 |
Table 6 Temporal experiments of 30 mM H2O2 concentration and various Fe+2 concentration
|
Time (Minute) |
5 mM Fe+2 15 mM H2O2 |
5 mM Fe+2 30 mM H2O2 |
5 mM Fe+2 45 mM H2O2 |
5 mM Fe+2 60 mM H2O2 |
|
5 |
5,01 |
8,03 |
16,02 |
17,29 |
|
15 |
7,43 |
10,95 |
15,04 |
16,31 |
|
30 |
4,52 |
12,89 |
25,04 |
16,32 |
|
45 |
3,06 |
20,68 |
25,29 |
16,8 |
|
60 |
5,24 |
33,82 |
18,22 |
27,29 |
|
90 |
10,60 |
34,31 |
19,92 |
16,31 |
Table 7 Temporal experiments of 5mM Fe+2 and various H2O2 concentration
Assesment of hydrodynamic cavitation/Fenton process
Hydrodynamic cavitation experiments were carried out following the conclusion of the experimental analysis, which determined the concentrations of Fe+2 and H2O2. We compared HC alone, HC+H2O2, HC+Fenton, and Fenton alone.
In this study, it was concluded that 30 mM of H2O2 dosage for 5 mM Fe+2 was the most suitable values for the pollutant removal of the leachate. 35.98% removal rate at 30 mM H2O2 dosage and 36.41% at 90 mM H2O2 dosage were obtained. Therefore, excessive dosing of H2O2 was found to be unnecessary for removal efficiency.
After determining the 5 mM Fe2 + and 30 mM H2O2 dose, a series of experiments were performed to determine the optimum pH value was obtained. According to the results, the optimum value of pH is 3, 5-4. This situation is also in line with the literature findings. With this objective, Fenton + cavitation system experiments were carried out for 90 minutes for Cv = 0.221 (35 L / min, Tort = 45 oC and orifice diameter = 5 mm) in addition to the specified conditions.
Table 7 shows that the Fenton assisted cavitation system has a higher COD removal efficiency than the cavitation system alone. Cavitation + 5.0 mM Fe2+ + 30 mM H2O2 increased to 44.28%, while 32.85% removal efficiency was achieved with 5.0 mM Fe2 + + 30 mM H2O2 90 min (Table 8).28
|
Applied process |
% COD removal rate |
|
Cavitation Alone (90 min., Cv=0.221) |
7 |
|
5.0 mM Fe2+ + 30 mM H2O2 |
32.85 |
|
Cavitation ((90 min., Cv=0.221) + 5.0 mM Fe2+ + 30 mM H2O2) |
44.28 |
Table 8 Applied processes and COD removal rate
The findings show that, when the suggested treatment strategy is used, hydrodynamic cavitation can offer low chemical consumption, high treatment efficiency with less energy, and the ability to operate in a compact reactor volume with rising reaction speed. When the Fenton oxidation and cavitation processes are performed together rather than individually, higher efficiency have been reported.
This study was financially supported by The Scientific and Technological Research Council of Turkey (TUBITAK) (Project No. 113Y425).
TUBITAK.
Authors declare that there are no conflicts of interest.

©2024 Dursun, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.