Journal of
eISSN: 2475-5540


Review Article Volume 10 Issue 1
Department of Biological Sciences, College of Science, Clemson University, USA
Correspondence: Dr. Vincent S Gallicchio, Department of Biological Sciences, 122 Long Hall, College of Science, Clemson University, Clemson, SC, USA
Received: April 21, 2025 | Published: May 15, 2025
Citation: Shenouda K, Gallicchio VS. Stem cell therapy as a method of treatment for Alzheimer’s disease. J Stem Cell Res Ther. 2025;10(1):72-77. DOI: 10.15406/jsrt.2025.10.00186
Alzheimer’s disease (AD) is a growing social, economic, and medical crisis, affecting millions and contributing significantly to healthcare costs globally. As the most common form of dementia, AD is characterized by progressive neurodegeneration. The neuron and synaptic loss that occurs in the diseased brain leads to several adversities such as cognitive decline, memory loss, learning disabilities, and mood disturbances. Given the irreversible nature of neural death in AD, therapeutic strategies focusing on neurogenesis and synaptogenesis offer very promising avenues for intervention, as they can replace rather than repair neurons and synapses. More specifically, these strategies facilitate the formation of new neurons in the brain and the creation of new synaptic connections between neurons. In particular, stem cell therapy presents itself as a prospective solution by promoting the regeneration of depleted neuronal circuitry, addressing the root cause of neurodegeneration. It is vital to understand the mechanisms underlying AD progression and explore innovative treatment options based upon them in order to mitigate the devastating impact of this disease. This report will highlight the budding potential of stem cell therapy mechanisms as a practical approach to treating the catastrophic affliction that is AD.
Keywords: alzheimer’s disease, genetic mutation, degeneration, stem cells, therapy
AD, alzheimer’s disease; Aβ, amyloid-beta; ESCs, human embryonic stem cells; HUSBCs, monocytes derived from human umbilical cord blood; iPSCs, induced pluripotent stem cells; MSCs, mesenchymal stem cells; NSCs, neural stem cells; PSAPP, presenilin-1/ amyloid precursor protein; sAPPα, soluble amyloid precursor protein alpha
AD is one of the most concerning ailments of our time, as it has continuously been ranked as one of the top 10 causes of death worldwide,1 emphasizing the pressing nature of a solution. There are currently over 55 million people worldwide living with Alzheimer’s,2 accounting for a grand global cost of care of $1.3 trillion per year.3 This further amplifies the malignant effects of this fatal clinical disorder. However, before exploring potential treatments, it is crucial first to understand the underlying causes of AD. Researchers have hypothesized many risk factors that are believed to play a role in causing Alzheimer’s. These include genetics, aging, environmental factors, and behaviors or habits.4 Although some risk factors are set in their influence, there is room for improvement for others. It is essential to be aware of possible lifestyle changes that can help prevent the onset of the disease as much as possible, however, this cannot always be done.
Familial Alzheimer’s is a result of a genetic mutation passed down through one’s family. It is a small portion of overall Alzheimer’s cases, estimated to be less than 5%.5 In these cases, symptoms begin to appear as early as 30-40 years old, otherwise known as “Early-onset familial Alzheimer’s disease (EOFAD)”. Three genes have been associated with EOFAD:
If a mutation occurs in any of these three genes, it follows an autosomal dominant pattern of inheritance. This results in each offspring having a 50% chance of inheriting the mutation.5
Nearly all Alzheimer’s cases involve the degeneration of an anatomical pathway, specifically the progressive breakdown of the brain's neuron network. Protein accumulation and misfolding are major contributors to this (Figures 1–3).
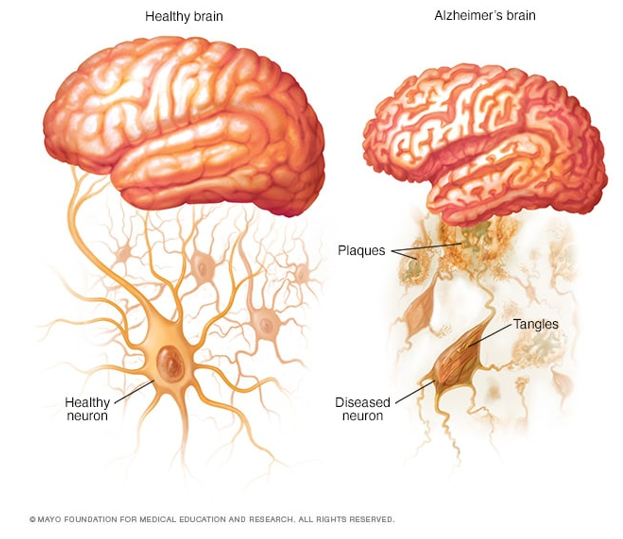
Figure 1 Visualization of amyloid beta (Aβ) peptide accumulation by comparing a healthy brain, containing no Aβ plaques, and an Alzheimer’s Brain containing Aβ plaques.6
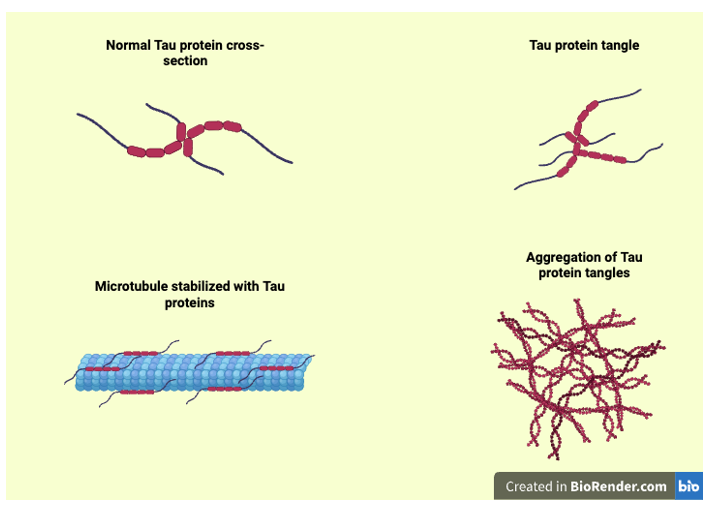
Figure 2 Aggregation of Tau Protein. Personal creation with the use of BioRender. Illustration depicts the physical appearance of normal Tau function when stabilizing microtubules, compared with Tau tangles and aggregates.7
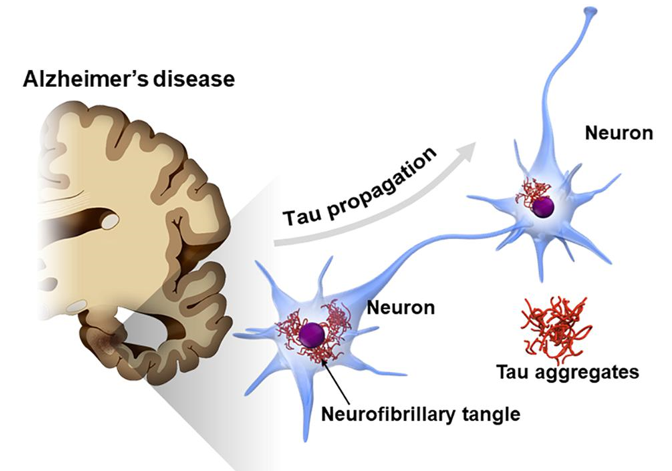
Figure 3 Visualization of the consequence of hyper phosphorylation of the Tau protein. As Tau protein aggregates, microtubules disassemble and disrupt cellular function, contributing to neurodegeneration associated with AD.8
Microglial cells, the brain's resident immune cells, undergo an initial activation that serves a protective function by attempting to clear Aβ plaques. This protective function is anti-inflammatory; however, when its activity fails or is prolonged, it can further damage the neurons and exacerbate inflammation. This releases cytokines, such as TNF-α and IL-1β, which harm neurons and further contribute to the progression of Alzheimer’s.9
Numerous environmental and lifestyle factors have been linked to the development of AD. Addressing these factors can reduce the risk of developing the disease, so it is essential to recognize them and take preventive measures accordingly.
Research has shown that regular physical activity enhances blood flow to the brain, promotes neuroplasticity, and may reduce the accumulation of amyloid plaques and tau tangles.10
Stress and depression can contribute to AD. This is because many biological changes occur in the brain, including the release of hormones such as cortisol, which can damage brain cells in the hippocampus. This results in a significant cognitive decline, accelerating the development of AD pathology.16
Found in fatty fish and flax seeds
Help reduce neuroinflammation and improve neuroplasticity.19
Protect neurons from oxidative stress, a significant contributor to AD progression.
Found in berries, dark chocolate, green tea, and vegetables.20
These things considered, it is certain that AD is very complex and can arise from an array of risk factors. Knowing such factors is essential when studying possible cures for this incurable disease. Stem cell therapy offers a promising treatment for these factors that are challenging to combat, as it has the ability to target the underlying biochemical pathways that lead to AD. By aiming to replace damage that results from the previously discussed risk factors, stem cell therapy has the potential to provide beneficial treatment for AD patients and change the lives of many who are suffering.
Stem cell therapy techniques rely on the understanding of how cells develop into new neurons and how these neurons integrate into the brain. There are two different ways stem cells undergo regeneration. The first of which is endogenous regeneration, the body’s natural ability to regenerate and repair damaged tissue. This category of regeneration is a natural restoration of function. The hippocampus, a critical brain region responsible for learning, memory, spatial navigation, and emotional regulation, illustrates this process. Approximately 1 million neurons are lost in the dentate gyrus and 5 million in the CA1 region of the hippocampus.20 Neuronal loss in this brain region results in significant challenges and early signs of Alzheimer’s, including short-term memory loss and disorientation. Yet, endogenous regeneration occurs to combat this loss of neurons, and around 700 new neurons are developed each day.21 This demonstrates the effectiveness of endogenous regeneration in replacing damaged tissue. However, it does have its limitations. One of these limitations is that the number of neurons developed daily declines with age, contributing to the memory loss associated with old age. The second classification of regeneration is engrafted regeneration, also known as exogenous regeneration. This involves the transplantation of stem cells into damaged tissue to stimulate the regeneration, repair, and functional restoration of the tissue. This can be done through several major groups of stem cells, as described in the Table 1 below.
|
Stem cell type |
Source |
Potency |
Key characteristics |
|
Embryonic stem cells (ESCs) |
Inner cell mass of a developing blastocyst (5–6-day embryo) |
Pluripotent: can differentiate into cells from the ectodermal, mesodermal, and endodermal germ layers. |
ESCs have an infinite ability for self-renewal, high differentiation potential for various cell types. |
|
Neural stem cells (NSCs) |
Brain |
Multipotent: can differentiate into various neural cell types. |
NSCs are responsible for neural development, allowing for neurogenesis and repair within the central nervous system. |
|
Induced pluripotent stem cells (iPSCs) |
Reprogrammed adult somatic cells |
Pluripotent |
iPSCSs are derived from patient’s own cells, which reduces the risk of immune rejection. |
|
Mesenchymal stem cells (MSCs) |
Various tissue types (bone marrow, adipose tissue, placenta) |
Multipotent |
MSCs promote healing through secretion of growth factors, modulate immune response, and migrates to injury sites. |
Table 1
Summary of major stem cell types, their developmental potency, and defining characteristics22
All of these major groups of stem cells have beneficial effects on Alzheimer's patients and can be utilized for various purposes. However, it could be inferred that NSCs would be best for directly replacing lost hippocampal neurons and rebuilding neural circuits. Additionally, iPSCs are also a promising option, as they significantly reduce the risk of immune rejection. Combining these two stem cell types would be the optimal approach for neuron regeneration in the brain. The next beneficial quality of stem cell therapy in the context of AD is its ability to promote neuroprotection. Reducing inflammation is crucial for promoting the survival of newly generated neurons and enhancing synaptic plasticity. The stem cell type most suited for the protective role is MSCs, which reduce inflammation and support neuronal survival through the secretion of growth factors. All in all, a combination therapy of iPSCs, NSCs, and MSCs would be the most effective treatment for regenerating neurons and protecting the brain.22
As previously mentioned, using these stem cells can alter key biochemical pathways that ultimately lead to AD. Again, these crucial pathways are the production of Aβ plaques and abnormal tau protein levels.
Aβ plaque reduction using stem cells
Mesenchymal stem cells (MSCs) can lower the rate of production of Aβ by reducing the expression of enzymes involved in Aβ synthesis. Stem cells can activate the brain’s immune cells, microglia, which can remove Aβ plaques through phagocytosis, a process where cells engulf and digest waste, in this case, Aβ plaques.23
Preventing tau tangles using stem cells
MSCs are also effective stem cells for preventing tau tangles. As previously mentioned as an AD risk factor, this increase in tau phosphorylation is detrimental because it causes neurofibrillary tangles, a key characteristic of an Alzheimer’s-affected brain. MSCs can inhibit tau protein aggregation by secreting proteins, such as Galectin-3, which plays a vital role in regulating inflammation, and thereby reduce tau phosphorylation.24
Previous studies have been conducted to assess the efficacy of stem cell therapy for AD patients.
Animal studies
Human studies
As of March 2025, Longeveron announced significant success with the results of its Phase 2a clinical trial, which evaluated the effect of Laromestrocel in AD patients. The results demonstrated improved cognitive function, quality of life, and brain volume in patients treated with the product. This clinical trial data supports the safety and efficacy of stem cell therapy as a treatment for AD.31
Although numerous preclinical studies in mouse models have demonstrated the therapeutic potential of stem cells for reducing cognitive decline and neuropathology in AD, human research remains in its early stages. Yet, initial clinical trials and case reports suggest stem cell therapies may offer promising benefits and have the potential to be valuable to the lives of those suffering from AD. To fully evaluate the method's safety, efficacy, and long-term outcomes in human patients, further large-scale studies are necessary.
Several key factors regarding stem cell therapy are essential to understand when considering treatment for AD patients, including the method of administration, timing of therapy, and the parameters by which success is measured. These are crucial in addressing both the safety and efficacy of using stem cells as a treatment for AD.
Stem cell administration
The method of administering stem cells for stem cell therapy varies case by case, but follows the following general protocol (Figure 4):
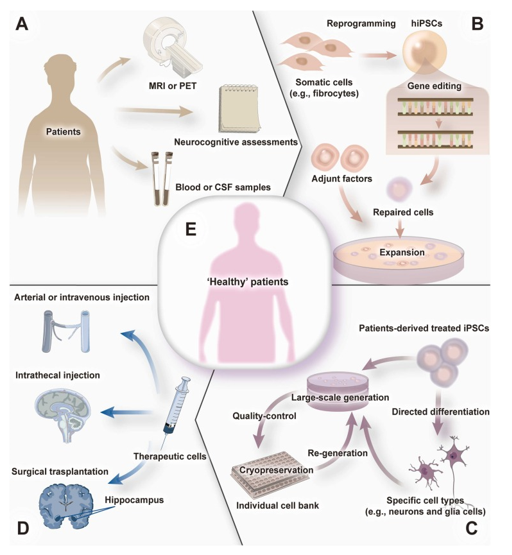
Figure 4 An illustration of the general stem cell administration procedure for neurodegenerative diseases.32
Timing of stem cell administration
The next facet that must be addressed is the importance of the timing of the stem cell administration. Research suggests that earlier intervention, when dementia symptoms are first recognized, may lead to better outcomes. This is because neuronal loss and synaptic degeneration are progressive processes that occur over time. Therefore, earlier treatment would help slow these adverse effects. More recently, studies have made significant advancements in the ability to detect AD in its early stages. Researchers from the German Center for Neurodegenerative Diseases have found that there is a specific biomarker of AD that is exhibited as early as 11 years before hallmark AD symptoms arise. This biomarker is characterized by an increase in beta-synuclein levels in the blood, a protein associated with neuronal damage and dysfunction. The protein is primarily found in synapses in the brain, and an increase in its presence is indicative of synaptic degeneration, a key contributor to AD. Studies have shown that beta-synuclein levels are elevated in AD patients from quite early on, even in the preclinical stages. Detecting beta-synuclein can lead to an earlier diagnosis of AD, which enables earlier intervention. In this context, it could help identify patients who would benefit from stem cell therapy and, hopefully, improve their health outcomes. This new finding is groundbreaking, as it plays a crucial role in determining the timing of stem cell administration, allowing for treatment to be provided much earlier when there is less neuronal degeneration. The timing of therapy in AD patients is an essential factor in the success of the treatment process.
Furthermore, studying levels of beta-synuclein can help monitor the progression of the disease, even after therapeutic interventions have been implemented. For instance, if levels of beta-synuclein decrease after treatment, that could be a sign of a positive treatment response. Before this finding, AD has been difficult to diagnose, requiring procedures such as PET scans or lumbar punctures. However, a blood test measuring beta-synuclein levels serves as a much less invasive alternative. The discovery of beta-synuclein as a blood-based biomarker for AD offers a promising step towards earlier, easier, and more precise diagnosis of this disease.33
Parameters of success following stem cell therapy
Key factors in determining whether stem cell treatment is successful in AD patients include:
Clinical trials must track these metrics to assess efficacy and safety over extended periods of time.
Summary: endogenous vs. exogenous cell regeneration
Both endogenous and exogenous regeneration prove useful in treating AD, each for in its own distinct reasons and ways. The Table 2 below provides a comprehensive overview of endogenous and exogenous regeneration.
|
Factor |
Endogenous regeneration |
Exogenous regeneration |
|
Mechanism |
Stimulation of hippocampus brain region with the use of patient’s own neural stem cells (NSCs) |
Introduces new cells (MSCs, iPSCs, NSCs) to replace lost or damaged neurons. |
|
delivery method & invasiveness |
Pharmacologically or genetic stimulation, Less invasive |
Injection via the brain or intravenous delivery, More invasive |
|
Immune response |
Minimal as it uses the body’s own cells |
Higher risk of immune rejection |
|
Challenges |
May not be powerful enough to combat the rapid age- related decline neuron regeneration |
Cost, integration, diseased environment of a brain with AD may impair transplanted cells from functioning. |
|
Applications |
Early stages of AD (mild degeneration) |
Advanced stages of AD (severe degeneration) |
Table 2 An organized comparison of endogenous and exogenous methods of cell regeneration21
AD remains a significant challenge worldwide to this day. It is an incurable disease that affects millions of individuals and their families, imposing both societal and economic burdens. However, it does not need to remain this way; although there is no current cure that does not mean there is no hope. The use of stem cell therapy presents a favorable opportunity to slow the progression of the disease and improve the quality of life for those affected by it. As previously discussed, this can be achieved through endogenous and exogenous neuronal regeneration strategies. The endogenous approach aims to stimulate the brain’s natural repair mechanisms. However, limitations exist, including the decline in neurogenesis with increased age and widespread neuronal loss. Exogenous therapies, on the other hand, utilize transplanted stem cells, such as MSCs, iPSCs, ESCs, and NSCs, to replace lost neurons, regulate inflammation, and restore cognitive function. Yet, challenges remain, including immune rejection, the high cost of treatment, and the scarcity of human clinical studies.
Again, despite these challenges, hope persists. Recent discoveries in the field include the newfound ability to derive cholinergic neurons, which degenerate and die when sick with Alzheimer’s, from umbilical cord-derived MSCs. Scientists have discovered a method to achieve this using efficient and cost-effective approaches, underscoring the potential of stem cell therapy in treating Alzheimer’s.35
As research progresses, it is essential to consider neuronal regeneration strategies, specifically the promising avenue of stem cell therapy, while addressing their limitations. This is crucial for developing the most effective treatments possible and gradually alleviating the burden and stress that many suffer from. By continuing to explore the potential of stem cell therapy as a method of treatment for Alzheimer’s patients, we are one step closer to making breakthroughs in combating this incurable disease.
None.
The authors declare that there are no conflicts of interest.

©2025 Shenouda, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.