Journal of
eISSN: 2475-5540


Review Article Volume 10 Issue 1
Department of Bioengineering, University of California, Los Angeles (UCLA), USA
Correspondence: Dr. Bill Tawil, Department of Bioengineering, UCLA School of Engineering, 420 Westwood Plaza, Room 5121, and Engineering V. P.O. Box: 951600, Los Angeles, CA 90095-1600, USA
Received: June 19, 2025 | Published: July 18, 2025
Citation: Kyra S, Bill T. Reconnecting the spine: a review of repair, regrowth, and rehabilitation. J Stem Cell Res Ther. 2025;10(1):145-152. DOI: 10.15406/jsrt.2025.10.00198
Spinal cord injuries, or SCIs, are life-altering traumas that disrupt motor, sensory, and autonomic function, often resulting in paralysis. Affecting millions globally, SCI lacks a definitive cure, driving the development of innovative therapies and devices. This report compares healthy and diseased tissue within the spinal cord and explores the current status of SCI treatment and market trends, including segmentation, emerging clinical trials, and established products like neuroprostheses, stem cell therapies, and spinal surgeries. Additionally, this paper aims to summarize the demographics, economic impact, and therapeutic landscape of SCI treatment in both commercial and clinical trial settings.
Keywords: spinal cord injury, electrical stimulation, stem cell therapy, tissue engineering, neurodegenerative disease
SCI, spinal cord injury; CAGR, compound annual growth rate; FES, functional electrical stimulation; BCI, brain computer interfaces
Spinal cord injuries are devastating, often leading to complete or partial neurological deficits across the world. Officially, they are defined as damage to the spinal cord that causes changes in its function either temporarily or permanently. This can cause loss of motor functions, bladder dysfunction, muscle spasticity, chronic pain, urinary tract infections, pressure ulcers, respiratory complications, osteoporosis, and deep vein thrombosis. In extreme cases, it can result in complete paralysis, loss of motor and sensory function, or death. There is no effective cure at the moment, with the typical mode of treatment being emergency surgery followed by months of physical therapy. Due to this, along with a rise in motor vehicle accidents and the geriatric population, the market is growing significantly. Current treatments include stem cell therapies, neuroprostheses, electrical stimulation, and surgery.
Healthy tissue anatomy
The spinal cord is a cylindrical tube of tissue responsible for controlling voluntary muscles of the trunk and extremities. Similar to the brain, it is composed of both grey and white matter, but contains grey matter on the inside and white matter externally.1 Grey matter contains neurons and glia, while white matter holds nerve fibers.2 With this structure, the spinal cord is able to receive information from the brain and transfer it to peripheral nerves, acting as the means of communication within the body. Additionally, it is responsible for sensation, autonomic, and motor control, including proprioception, reflexes, response to stimuli, the ability to touch, determine pain, and sense temperature.3
The spinal cord starts at the bottom of the brainstem and runs along the spine, ending in the conus medullaris, a conical structure in the lower back. In a healthy adult, it is typically 45 cm long and 1 cm thick. The spinal cord is divided into multiple segments along the spine - eight cervical, twelve thoracic, five lumbar, five sacral, and one coccygeal. In total, these segments contain 31 nerves, of which 30 are in pairs with one on each side. The cervical segment has eight nerve pairs that extend mostly to the face and head, the thoracic segment contains twelve nerve pairs that branch into the chest, upper back, and abdomen, and the lumbar segment contains five pairs going to the legs and feet. Additionally, there are five sacral nerve pairs in the lower back that run to the pelvis. The final nerve bundle is called the cauda equina, which provides sensation to the lower body.4 The segmentation of the spinal cord and the nerve distribution is shown in Figure 1.
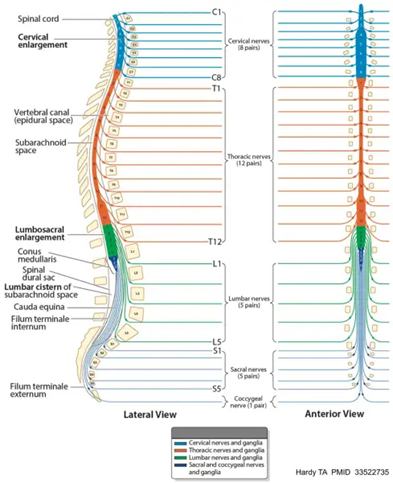
Figure 1 Spinal cord anatomy.
The image shows the anatomy of the spinal cord, including the various segmentations and pairing of nerves.
Furthermore, the spinal cord is covered in layers of protective tissue called meninges which include the dura mater, arachnoid mater, and pia mater. It also houses various cell types, which can be broadly summarized as neurons and glial cells. On a micro level, neurons are further divided into motor, sensory, and interneurons which change their function based on the input received. Glial cells can be categorized into astrocytes, oligodendrocytes, ependymas and microglias. Astrocytes guide migration of neurons and act as scaffolding in the central nervous system, while oligodendrocytes are responsible for producing myelin. Ependymal cells line the central canal of the cord and help circulate cerebrospinal spinal fluid. Finally, microglia are the immune cells within the CNS and remove debris through phagocytosis.3
Healthy tissue blood supply
The spinal cord primarily receives blood from the anterior spinal artery and two posterior spinal arteries. The anterior spinal artery provides the anterior two thirds of the cord with blood and runs along the ventral surface. The posterior third is supplied by two posterior spinal arteries composed of paired vessels that run along the dorsal surface of the cord. In addition to these, the spinal cord contains radicular and medullary arteries as well as anterior and posterior veins that drain blood.5 The arterial blood supply of the spinal cord is shown in Figure 2.
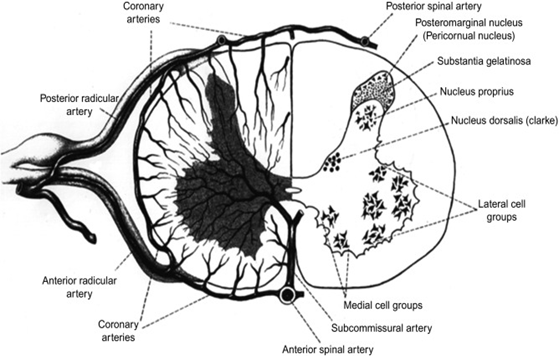
Figure 2 Transverse section depicting arterial blood supply of spinal cord.
The image shows the arteries that supply the spinal cord with blood as well as the cell groups that exist within the cord.
Diseased tissue
Spinal cord tissue can be damaged due to multiple diseases, including infectious, autoimmune, neurodegenerative, traumatic, congenital and neoplastic.5 Examples of these are shown in Table 1.
|
Disease |
Description |
Symptoms |
|
Infection |
||
|
Spinal epidural abscess58 |
Pus between the dura mater and vertebrae |
Back pain, fever, weakness, numbness, spinal compression, paralysis |
|
Vertebral osteomyelitis56 |
Infection of vertebral bones caused by spread of bacteria |
Back pain, fever, tenderness of spine, neurological deficits |
|
Autoimmune |
||
|
Guillain-barre syndrome51 |
Acute autoimmune attack on peripheral nerves |
Weakness, tinging, possible respiratory effects |
|
Multiple sclerosis51 |
Autoimmune demyelination of neurons in the central nervous system |
Weakness, sensory issues, fatigue |
|
Transverse myelitis57 |
Inflammation across a segment of the spinal cord |
Sudden paralysis or weakness, sensory loss, urinary retention |
|
Neurodegerenative |
||
|
Amyotrophic lateral sclerosis58 |
Progressive degeneration of motor neurons |
Muscle weakness, spasticity, respiratory failure, dysphagia |
|
Spinal muscular atrophy59 |
Degeneration of lower motor neurons |
Symmetrical muscle weakness, poor motor development |
|
Nerve root disorders |
||
|
Radiculopathies60 |
Nerve root irritation or compression |
Pain, numbness, tingling, weakness |
|
Mechanical / Traumatic |
||
|
Herniated disk51 |
Disk protrudes and compresses nerves |
Back and neck pain, possible arm or leg pain, motor deficits |
|
Cervical spondylosis61 |
Degeneration of cervical spine joints and discs |
Neck stiffness, pain, myelopathy |
|
Congenital |
||
|
Spina bifida62 |
Incomplete closure of neural tube during fetal development |
Leg paralysis, hydrocephalus, bladder or bowel disfunction |
|
Neoplastic |
||
|
Spinal cord tumors63 |
Abnormal growths inside or near spinal cord |
Progressive weakness, sensory loss, pain, paralysis |
|
Meningioma64 |
Tumors from meninges that can compress spinal cord |
Gradual weakness, sensory loss, coordination issues |
Table 1 Types of spinal cord damage
The most frequent mechanism of spinal cord injury is spinal cord compression. This can lead to local hemorrhages, ischemia, and hypoxia, causing an interruption of blood supply and depletion of oxygen in tissues. This damages the grey matter in the cord, also fracturing neurons in the damaged area and reducing the thickness of myelin present. Similarly, white matter damage happens through axonal degeneration and demyelination.6 Additionally, spinal cord injury typically results in an inflammatory response from the immune system, including the release of inflammatory cytokines and changes in microglia. When the central nervous system is damaged, microglia change their morphology from branching to a rounded cell body and begin cleaning debris as in addition to secreting healing molecules. They also begin to proliferate extensively around the site of injury. Astrocytes become reactive as well in a process called astrogliosis, resulting in a glial scar which stabilizes tissue but may impede axonal growth.7
Over time, inflammation and chronic injury caused from spinal cord damage can lead to spinal cord atrophy.8 Unfortunately, there is no current treatment that can regrow axonal fibers after a complete spinal cord injury. This is primarily due to a lack of an axonal supporting microenvironment.9
Spinal cord injury is a leading cause of paralysis and neurodegenerative effects in the United States and around the world.10 In the US in 2021, there were 54 cases per 1 million people, with an estimation of around 17,810 new cases every year.11 The primary cause of spinal cord injuries are motor vehicle crashes, but also includes violence, sports, recreational activities, tumors, or chronic diseases.12
Globally, around 15.4 million people were living with spinal cord injuries in 2021.13 As compared to just the United States, this ranges from 10.4 to 83 cases per million people per year.11 Additionally, the global spinal cord injury treatment market size was 7.25 billion USD in 2024, and is projected to reach 10.72 million by 2032 with a CAGR of 5.1%.14 North America has the largest market size, with the Asia-Pacific region being the fastest growing. This is due to a rising prevalence in road accidents, as well as a rising geriatric population and increased need for updated medical technology. Major players in the medical technology field are Medtronic, Stryker, Johnson and Johnson, Zimmer Biomet, Novartis AG, Tenax Therapeutics, Accrue Pharma, and Kringle Pharma.15,16
The global market as well as its projected increase are displayed in Figures 3 and 4.
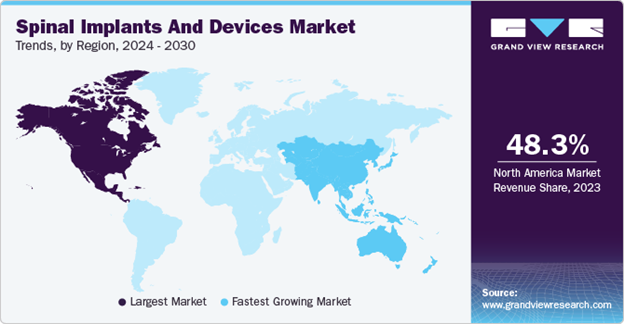
Figure 3 Spinal implant and device market trends by region.
The image shows the spinal implant and device market trends by region from 2024 to 2030, illustrating the largest market is in North America.
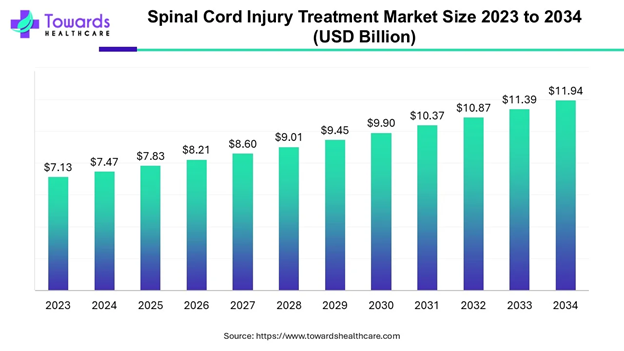
Figure 4 Projected increase in global spinal cord injury market.
The image shows the projected increase in the global spinal cord injury market, highlighting an overall increasing trend until 2034.
SCI affects males more than females; since 2015, approximately 79% of new cases have been male. Additionally, the average age has increased from 29 in the 1970s to 43 since 2015.17 Older people are more susceptible to spinal cord injuries, due to their increased likelihood of falling as well as an increased prominence of neurodegenerative disc disease.18 That said, spinal cord injuries primarily affect young adults between the age of 16 and 30. This is shown in Figure 3a and 3b, which describe the incidence and prevalence rate respectively. Figure 3 highlights the dominance of SCI’s in men for those under 70.
Economically, costs incurred due to spinal cord injury vary significantly based on severity. In the first year following the injury in the U.S, less severe traumas ranged from $111,780 to $300,880 while severe injuries could cost up to $1,156,410. After the first year, less severe injuries cost around $4,490 annually and severe injuries cost $251,450 per year. Furthermore, total lifetime costs range from $500,000 to over $1 million.19 The total annual direct costs associated with SCI in the U.S. are estimated at approximately $7.7 billion, with motor vehicle accidents being the largest contributor.20
Market segments
The market is segmented into various categories to better guide R&D decisions and make product decisions.14,21 This division is summarized in Table 2.
|
Category |
Segmentation |
Description |
|
Treatment type |
- Non-operative care - Operative care |
Non operative care: rehabilitation, medications.
Operative care: surgery, implantable devices |
|
Injury type |
- Complete - Partial |
Partial cord injury offers a higher chance of recovering function and less treatment |
|
Injury severity |
- Cervical - Thoracic - Lumbar - Sacral |
Cervical is the most severe and most expensive |
|
Patient characteristics |
- Gender - Age |
Spinal cord injuries are most common in males, young adults, and the geriatric population |
|
End user |
- Hospital - Trauma centers - Clinics - Research centers |
Different facilities offer different functions, including acute care, outpatient care, or rehabilitation |
|
Region |
- United States - EU-4 (Germany, France, Italy, Spain) and the United Kingdom - Japan |
America has the largest market, but the Asia-Pacific region is the fastest growing due to a rise in elderly population and increased demand for medical technology. |
Table 2 Market segmentations
Although there is no cure for spinal cord injuries, there are existing treatments and therapies designed to maximize the patient's quality of life. These can be broadly categorized into three groups; stem cell therapies, neuroprostheses and electrical stimulation, and surgery.22
Stem cell therapies
Stem cells are able to differentiate into any type of cell within the body, so by using them as a treatment method, researchers aim to replace lost neurons, remyelinate axons, and promote growth to stimulate recovery. However, they come with an increased risk of cancer or immune rejection.23 Many studies exist evaluating their potential as treatment, but a Japanese company called Nipro Corp was able to put an approved product on the market: Stemirac Inj. This has been conditionally approved by the Japanese PMDA in 2018 for supervised use. Stemirac uses autologous mesenchymal stem cells from bone marrow that travel to the site of injury and differentiate into new nerve cells. Early stage clinical trials showed neurological improvement in 12 out of 13 patients six months after the injection, as measured by the American Spinal Injury Association Impairment Scale (ASIA).24,25
Neuroprostheses and electrical stimulation
Neuroprostheses are devices that use electrodes to interact with the nervous system in order to restore motor and sensory function after spinal cord injury. They can stimulate various parts of the nervous system, including muscles, nerves, spinal cord, and the brain. These are separated into functional electrical stimulation (FES) or brain computer interfaces (BCIs), and can be implantable or not. A comparison of each category, along with relevant products and companies, are summarized in Tables 3 and 4.26-28
|
Product |
Company |
Description |
Image |
Year released |
|
Freehand system24 |
Neuro control |
8 channel receiver stimulator system designed to help hand motor control |
www.researchgate.net/figure/nd-Generation-FreeHand-system_fig3_51113721 |
1997 |
|
IST-1225 |
Neuro control |
Second generation of freehand system; 12 channels, also aimed towards hand function |
https://doi.org/10.1109/tnsre.2010.2079952. |
2000 |
|
ARC-EX26 |
Onward Medical |
Noninvasive stimulation geared towards improving hand function |
onward.athlon.london/therapy/arc-ex |
2024 |
|
Atrostim27 |
Atrotech |
Neurostimulator of phrenic nerves to stimulate respiratory response |
www.atrotech.com |
1998 |
|
NeuStim28 |
Neuvotion |
Wearable, non-invasive wrist neurostimulation device to recover hand movement
|
www.neuvotion-inc.com |
2025 |
Table 3 FES products
|
Product |
Company |
Description |
Image |
|
ARC-BCI26 |
Onward Medical |
Implantable device that uses artificial intelligence to translate thought into movement |
www.onward.athlon.london/therapy/arc-ex/ |
|
N129,30 |
Neuralink |
Implantable chip that sends brain signals to a device, allowing patients to control technology |
www.neuralink.com |
Table 4 BCI products
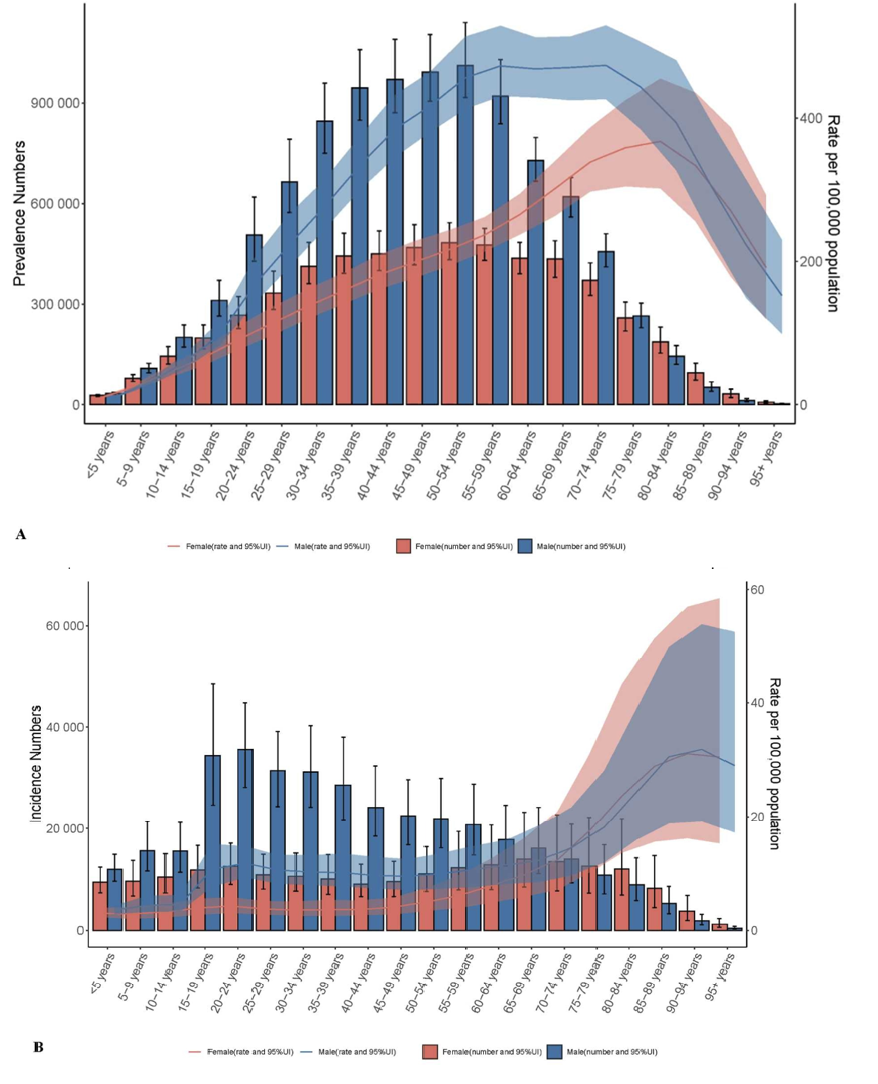
Figure 5 Age-sex correlation in SCI in 2021 in men and women.
The image compares the prevalence and incidence rates of spinal cord injuries in men and women, showing that it is more common in men.
In addition to neuroprosthetics that utilize electrical stimulation, treatments such as exoskeletons and braces exist to aid in rehabilitation following spinal cord injury. EksoNR by Eksobionics is the only FDA approved exoskeleton, and is used to teach individuals how to walk again following injury (Image).29
Surgery
In the United States, approximately 74% of individuals with spinal cord injury undergo early surgery.30 Surgery is typically done to either decompress the spinal cord or stabilize the vertebral bones and tissues surrounding the cord, in hopes of preserving as much neurological function.31,32 Various types and their functions are summarized in Table 5.
|
Procedure |
Type of surgery |
Description |
Advantages |
Limitations |
|
Laminectomy |
Decompression |
Removing the entire lamina |
Effectively reduces compression, good for stenosis or trauma |
Can reduce spinal stability |
|
Laminotomy |
Decompression |
Removing a section from the lamina |
Less invasive |
May not fully relieve all the pressure |
|
Discectomy |
Decompression |
Removing a portion of a vertebral disc |
Minimally invasive, relieves compression |
Still possible for the disc to degenerate |
|
Foraminotomy |
Decompression |
Removing some amount of bone to expand the nerve root opening |
Can preserve motion |
Risk of nerve damage, doesn’t address instability if present |
|
Expansile duraplasty |
Decompression |
Enlarging the dura to reduce pressure or swelling |
Can improve spinal cord perfusion |
Risk of CSF leaks |
|
Anterior cervical discectomy and fusion |
Stabilization |
Removing a cervical disc from the front |
High success rate, preserves alignment |
Reduces mobility at fused segment, risk of adjacent segment disease |
|
Posterior fusion |
Stabilization |
Fusing vertebrae using rods, screws, bone grafts from the back |
Strong stabilization |
Large incision, longer recovery, potential for muscle damage |
In addition to stem cell therapies, neuroprosthesis, electric stimulation, and surgery, spinal cord injuries are treated with rehabilitation and pain medication. A comparison of each method is summarized in Table 6.15,33
|
Treatment |
Advantages |
Limitations |
|
Stem cell therapies |
Potential to heal body by generating new neurons |
Highly experimental Risk of immune rejection or teratoma formation |
|
Neuroprostheses |
Can provide partial control of voluntary muscles to restore function Can be integrated with BCI’s or stimulation |
Surgical implantation Only useful for certain injury levels Expensive |
|
Electrical stimulation |
Enhances muscle function and strength Can be non-invasive |
Temporary effects Constant therapy required |
|
Brain-computer interfaces |
Allows direct communication between brain and devices Can essentially completely bypass injury |
Highly invasive and experimental Expensive |
|
Surgery |
Prevents further damage, restores stability Can be used for implantation |
Doesn’t reverse damage Risk of complications Can reduce mobility |
|
Rehabilitation |
Strengthens muscles and improves function |
Not a cure Requires constant effort and sessions Slow progress |
Table 6 Comparison of different treatments
Comparison of various treatment methods
Within the existing fields of treatment, innovations are being made to advance treatment of spinal cord injuries.34 In vivo therapeutic corporation has invented a neuro spinal scaffold that has been approved as a humanitarian use device by the FDA. This implant, made from PLGA, is designed to treat acute spinal cord injuries by providing structural support and promoting tissue healing. It is a porous biodegradable polymer that allows cells to pass through, and is meant to be implanted during decompression surgery days after getting injured. This was tested in the INSPIRE trial, and early results showed it was safe to implant. Additionally, it was implanted in a 25 year old male who reported improvements after 3 months.35
Additionally, an ongoing trial conducted by Mayo clinic is investigating the use of autologous adipose derived mesenchymal stem cells.36 Researchers harvested adipose tissue from the patient's abdomen or thigh, grew them in the lab, and then injected them back into the spinal canal approximately 4-6 weeks later. In doing so, they hoped to reduce inflammation and promote vascularization, ultimately making a friendlier environment for nerve regeneration within the spinal cord. Phase I of the trial was completed, and the treatment was determined to be safe. However, the results varied; some patients showed a significant increase, while others did not have a noticeable change in outcomes. Therefore, the goal of Phase II is to further investigate results and identify optimal treatment methods.37
Another emerging technology is being developed by NovaGo Therapeutics AG, and current clinical trials investigate the use of the human monoclonal antibody NG004. This drug aims to inhibit Nogo-A, which is a protein that restricts axonal growth within the nervous system. By inhibiting Nogo-A, researchers hope to promote axonal growth in patients with acute spinal cord injuries. NovaGo administered the drug to their first patient in January of 2025, and the study is estimated to be completed in November of 2026.38
Finally, Lineage Cell Therapeutics is developing an oligodendrocyte progenitor cell therapy called OPC1. These cells are designed to replace damaged cells in the spinal cord in hopes of restoring function. In Phase 1 of the trial, safety was established, allowing Phase 1/2a to be implemented, which showed that a year after receiving treatment, 96% of participants recovered at least one level of neurological function on one side of their body. Currently, the product is undergoing a DOSED study to evaluate the effects of administering the drug directly to the spinal cord.39
In addition to stem cell therapies, innovations are being made regarding brain computer interfaces and electrical stimulation.40-50 This report will not focus on them in depth, but an interesting BCI currently being tested is BrainGate’s chip, which aims to give the patient complete control over their surroundings instead of just device control. Moreover, the Spectra Wave Writer Spinal Cord Stimulator System tests the effect of epidural stimulation on sensorimotor and autonomic functions in chronic patients.39 This study is estimated to be completed in December 2025.51-57
Spinal cord injuries are a major challenge to public health, affecting millions of people globally.14 Incidence rates are expected to grow due to trauma and age, prompting a growth in the market size.15,58-68 Although there is no cure, progress has been made in developing therapeutic options that improve patient outcomes.42,69,70 Stem cell therapies offer regenerative potential, neuroprostheses and electrical stimulation provide functional restoration, and surgical techniques remain essential for acute management and device implantation.43 Additionally, innovation continues in the form of clinical trials, and continued investment and scientific research within this field could change how spinal cord injuries are treated in the future.32
There is no funding to report for this study.
Kyra Sunil expresses appreciation to Professor Bill Tawil for overseeing the framework of this review, and for the insightful lectures and advice concerning biomaterials and tissue engineering, which contributed to the development of this paper.
The authors declare that there is no conflicts of interest.
Companies
1a. Nipro Corp: https://www.nipro.co.jp/en/
1b. Neuro Control: https://tracxn.com/d/companies/neurocontrol/__HuNsXB5ULSnZFMT6SHnOCJBGAwo5QIWTJ4ZUBrAu-7g
1c. Onward Medical: http://onward.athlon.london/about/
1d. Atrotech: http://www.atrotech.com/
1e. Neuvotion: https://www.neuvotion-inc.com/
1f. Neuralink: https://neuralink.com/
1g. InVivo Therapeutic Corporation: http://www.invivotherapeutics.com/
1h. NovaGo Therapeutics AG: https://www.novagotherapeutics.com/
1i. Lineage Cell Therapeutics: https://lineagecell.com/
1j. BrainGate: https://www.braingate.org/
Ik. Eksobionics: https://eksobionics.com/
Products
2a. Freehand system: https://scireproject.com/evidence/upper-limb/complementary-and-alternative-medicine/freehand-system/
2b. IST-12: https://pubmed.ncbi.nlm.nih.gov/20876029/
2c. ARC-EX: https://onward.athlon.london/therapy/arc-ex/
2d. Atrostim: http://www.atrotech.com/products/Atrostim_PNS_V25
2e. NeuStim: https://www.neuvotion-inc.com/
2f. ARC-BCI: https://onward.athlon.london/brain-computer-interface/
2g. N1: https://neuralink.com/trials/
2h. EksoNR: https://eksobionics.com/eksonr/

©2025 Kyra, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.