Journal of
eISSN: 2475-5540


Review Article Volume 10 Issue 1
1Immorta Bio Inc, USA
2Plush Transportations LLC, USA
3Azimi Cardiovascular Institute, USA
4The Artful Move, USA
5Veltmeyer Institute, USA
6Loma Linda University, USA
7BioCentrium LLC, USA
Correspondence: Dr. Thomas E Ichim, President and Chief Scientific Officer, Immorta Bio, USA
Received: April 21, 2025 | Published: May 20, 2025
Citation: Ramos RA, Ichim TE, Agamirov A, et al. Mesenchymal stem cells for rheumatoid arthritis. J Stem Cell Res Ther. 2025;10(1):79-86. DOI: 10.15406/jsrt.2025.10.00187
Rheumatoid arthritis (RA) is an autoimmune disease characterized by the progressive erosion of the synovial membrane, destruction of cartilage, bone erosion, and, ultimately, the loss of joint functionality. Additionally, RA may exhibit various systemic effects that can cause severe disability. Although the introduction of biological disease-modifying antirheumatic drugs (DMARDs) has revolutionized the standard of care, a substantial proportion of the population do not achieve significant responses or develop resistance. Additionally, patients that do respond to DMARDs can have numerous adverse effects as well as limited ability to regenerate lost tissue. Here, we discuss the immunology of RA, how mesenchymal stem cells (MSCs) block the pathology of RA, and we provide an overview of animal and clinical studies supporting the use of MSCs in the treatment of RA. We also describe preliminary data using our proprietary pluripotent derived MSCs (pMSC) that can be utilized for induction of antigen-specific tolerance.
Keywords: rheumatoid arthritis, autoimmune disease, immunomodulation, regenerative therapy, joint inflammation, clinical studies, stem cell therapy
Rheumatoid arthritis (RA) is a chronic inflammatory disorder affecting approximately 0.5-1% of the global population,1 characterized by immune-mediated synovial inflammation and joint deterioration. Additionally, numerous extra-articular manifestations have been reported, including accelerated atherosclerosis, heart attacks, pericarditis, and pulmonary pathology.2
In general, because of the critical role of inflammation in the pathogenesis of RA, patients are usually started on non-steroidal anti-inflammatory drugs (NSAIDS); however, more recent practice has been the concurrent initiation of disease-modifying antirheumatic drugs (DMARDs). These agents are slow acting but have been demonstrated to inhibit the radiological progression of RA. Such agents typically include: 1) hydroxychloroquine, which acts in part as a Toll-like receptor (TLR) 7/9 antagonist,3 thus decreasing innate immune activation;4 2) leflunomide, an antimetabolite that inhibits pyrimidine synthesis and protein tyrosine kinase activity,5 leading to suppression of T cell responses,6 inhibition of dendritic cell (DC) activation7 and augmentation of T regulatory (Treg) cell activity;8-10 3) injectable gold compounds such as auranofin, which directly or through metabolites such as dicyanogold have been demonstrated to inhibit T cell and antigen-presenting cell activation,11,12 as well as cause T helper 2 (Th2) deviation;13 4) sulfasalazine, was used since 1950, and acts primarily through inhibition of cyclooxygenase and lipoxygenase;14 and 5) methotrexate, an antifolate that inhibits T cell activation and proliferation, that has been one of the golden standards for RA.15 Typically, combinations of DMARDs with glucocorticoids are used, or alternatively pulse of high-dose glucocorticoids are administered to cause a general inhibition of inflammation.16
The TNF-α targeting agents, Remicade, Enbrel, and Humira, sometimes referred to as “biological DMARDs” are used primarily after response to conventional DMARDs has failed.17 Although improvement in quality of life has occurred as a result of biological DMARDs, substantial progress remains to be made. For example, TNF-alpha blockers have been associated with reactivation of infectious diseases, autoantibody formation and the possibility of increased lymphoma risk.18-20 Thus, to date, one of the major limitations to RA therapy has been the inability to specifically inhibit autoreactive responses while sparing immune responses to other stimuli.
Because RA is driven by immunity against “self” antigens, the possibility exists of specifically inhibiting the pathological response through “reprogramming” of immune effectors in a manner allowing for selective silencing (and/or eliminating) of autoreactive clones. While current treatments for RA are non-cognate when it comes to antigen specificity, the possibility of inducing tolerance would literally be a cure for this disease without the side effects of ongoing immune suppression.
Immunological tolerance is the state of selective ignorance towards self, while maintaining intact ability to kill “non-self”. Tolerance induction, which may occur at the “central” or “peripheral” levels involves mechanisms that suppress or redirect immune responses against self-antigens,21 innocuous environmental antigens such as allergens,22 or transplanted tissues.23
Central tolerance is primarily established in the thymus for T cells and bone marrow for B cells, where autoreactive lymphocytes are eliminated or rendered non-functional.24
For T cells, thymic epithelial cells and dendritic cells present self-antigens in the context of major histocompatibility complex (MHC) molecules. Thymocytes (T cell precursors) with high-affinity TCRs are deleted via negative selection, which is executed by dendritic cells and macrophages present in the thymic medulla or at the cortico-medullary junction. In addition, thymic medullary epithelial cells (MECs) that ectopically express tissue-specific proteins under the control of a transcription factor called Autoimmune regulator (Aire) present such self-peptides to developing T cells with autoreactivity for such antigens. As a result, the latter are eliminated or skewed towards a regulatory phenotype.25,26
For B cells, central tolerance occurs through clonal deletion or receptor editing in the bone marrow. Autoreactive B cells encountering self-antigens undergo apoptosis or rearrange their B-cell receptor (BCR) genes to reduce self-reactivity.27 These processes ensure that only lymphocytes with low-to-moderate affinity for self-antigens exit to the periphery, minimizing autoimmune risk. Although studies are conflicting, it appears that Treg cells are needed for B regulatory cell generation and that B regulatory cells are not positively selected for in the bone marrow.28
Peripheral tolerance complements central mechanisms by controlling mature lymphocytes that escape central deletion, essentially acting as a “backup” system. Anergy, a state of functional unresponsiveness, is induced when T cells receive TCR stimulation (signal 1) without co-stimulatory signals (signal 2), such as CD28-B7 interactions.29 For example, in the absence of inflammation, dendritic cells presenting self-antigens in steady-state conditions induce T-cell anergy, preventing autoimmunity. Another key mechanism is the induction of Tregs, which maintain immune homeostasis by suppressing helper and cytotoxic T cells,30-32 B cells,33 and downregulating the ability of the dendritic cell to initiate immunity.34
Tregs are critical in mucosal tolerance, exemplified by oral tolerance, where ingestion of antigens (e.g., food proteins) induces Treg differentiation in gut-associated lymphoid tissues.35,36 This process, mediated by transforming growth factor-β (TGF-β) and interleukin-10 (IL-10), not only prevents food allergy but has been used therapeutically in a wide range of autoimmune models ranging from rheumatoid arthritis,37 to type 1 diabetes,38 to multiple sclerosis.39 Similarly, in transplantation, tolerance can be induced by co-stimulatory blockade (e.g., abatacept CTLA-4-Ig or anti-CD40L antibodies), promoting Treg expansion and anergy in alloreactive T cells,40 thus eliminating the need for chronic immunosuppressants by “educating” the immune system not to reject the foreign graft.
In autoimmune diseases like type 1 diabetes, antigen-specific immunotherapy aims to restore tolerance to autoantigens (e.g., insulin peptides). Low-dose administration of insulin peptides to at-risk individuals induces antigen-specific Tregs, reducing islet-specific T-cell responses.41,42 In allergy, allergen-specific immunotherapy (e.g., subcutaneous or sublingual administration of pollen extracts) desensitizes patients by skewing immune responses from Th2-driven IgE production to Treg-mediated IL-10 and IgG4 production, thus alleviating symptoms.43,44 In transplantation, mixed chimerism protocols, where donor hematopoietic stem cells are infused into recipients under non-myeloablative conditioning, establish long-term tolerance by deleting alloreactive T cells and promoting donor-specific Treg development.45
These examples underscore the potential of tolerance induction to rewire immune responses, offering durable solutions without the need for broad immunosuppression.
In conclusion, immunological tolerance induction is an ideal means of selectively “switching off” autoimmune attacks, such as those causing pathology in RA, while leaving other components of the immune system intact so as to not compromise the patient’s ability to fight infections and cancers.
To therapeutically implement antigen-specific tolerance-inducing strategies to RA, it is essential to have knowledge of autoantigens that are present in a majority of the patient population and contribute to disease. There are numerous antigens that have been determined or speculated to act as immunological targets of autoreactive T cells in RA which include citrullinated fibrinogen,46,47 aggrecan,78 vimentin,49 α-enolase,50 and filaggrin,51 as well as glucose-6-phosphate isomerase,52 hsp60,53 hsp70,54 and heterogeneous nuclear ribonucleoprotein A2.55 Of the known antigens, collagen II is perhaps the most studied. We will discuss this in detail with the understanding that other autoantigens exist and can similarly be used for induction of tolerance.
Collagen II is an extracellular matrix component found primarily in the synovial tissue that is usually sequestered from immunological attack. Induction of an RA-like disease has been reported in inbred murine strains following immunization of xenogeneic collagen II in the presence of adjuvant.56
Supporting a causative immunopathological effect of collagen II specific T cells were experiments in which the RA-like disease could be transferred to naïve recipients by administration of lymph node cells.57 Subsequent work cloning T cell lines from synovial membranes of patients with RA demonstrated existence of collagen II-specific T cells that persisted for a period of at least 3 years in vivo.58 Subsequent PCR-studies of the T cell receptor beta chains confirmed the oligoclonal expansion of collagen II-reactive cells in patients.59 In 1993, Weiner’s group reported a double-blind, placebo-controlled trial of 60 patients with advanced RA treated by oral administration of chicken collagen II for a period of 3 months. Responses in terms of decreased number of swollen joints were observed in the treated population but not in placebo controls. Of the patients treated, four presented with complete remission of disease. No treatment-associated adverse effects were noted.60 Unfortunately, Phase III trials using oral tolerance in RA have not met primary efficacy endpoints.61
Given the general failure of oral tolerance in RA, more specific approaches have involved stimulation of tolerogenic responses using ex vivo manipulated DC. Dendritic cells (DC) under physiological conditions promote tolerance, and when exposed to injury/damage signals mature and induce T cell activation. By ex vivo manipulating antigen-pulsed, donor-specific DCs, we have previously been able to induce antigen-specific suppression of immunity and generation of T regulatory (Treg) cells Tolerogenic modifications of DC performed by our group have included exposure of DCs to small-molecule immune suppressants,62-64 transfection with tolerogenic genes65-66 and silencing of immune stimulatory genes.67-70 In our previous work, we demonstrated the ability to prevent RA induction by pulsing immature DCs with collagen II (CII) and suppressing DC maturation with chemical or genetic means.71-74 Limitations of these data have been the lack of robust inhibition of inflammatory responses when administration of manipulated DC was performed at various time points subsequent to disease onset. The general failure of antigen-specific approaches, both in oral tolerance, as well as DC-based approaches may be the result of underlying inflammatory reactions. The advantage of utilizing mesenchymal stem cells as “living anti-inflammatory” agents is that the cells produce more anti-inflammatory agents the more inflammation is present. Additionally, the multiple mechanisms employed by MSCs may not only prevent inflammation but also stimulate regenerative processes. This is different than biological DMARDs, which reduce pathology but do not stimulate regeneration.
The possibility of using systemically-administered mesenchymal stem cells (MSCs) as a cellular therapy for RA has several conceptual advantages that address the previously mentioned drawbacks of current approaches. One such advantage is that the MSC may be viewed as a “smart” immune modulator. In contrast with currently available therapies, which globally cause immune suppression, production of anti-inflammatory factors by MSCs appears to be dependent on their environment, with upregulation of factors such as TGF-beta, HLA-G, IL-10, and neuropilin-A ligands galectin-1 and Semaphorin-3A in response to immune/inflammatory stimuli but little in the basal state.75-79 Additionally, systemically administered MSC possess the ability to selectively home to injured/hypoxic areas by recognition of signals such as HMGB1 or CXCR1, respectively.80-83
The ability to home to the sites of injury, combined with selective induction of immune modulation only in response to inflammatory/danger signals, suggests the possibility that systemically administered MSC do not cause global immune suppression.84-85 This is supported by clinical studies using MSCs for other inflammatory conditions, which to date, have not reported global immune suppression associated adverse effects.86-88 Another important aspect of MSC therapy is the ability of these cells to regenerate injured tissues through direct differentiation into articular tissue,89 as well as their ability to secret growth factors capable of augmenting endogenous regenerative processes.90
Physiologically, the role of MSCs in RA is a matter of debate. Nakagawa et al.,93 used radiolabeling of bone marrow cells to demonstrate migration of bone marrow stromal cells into the synovium of rats suffering from collagen-induced arthritis (CIA). While inference was made to contribution of the MSC to synovial proliferation, a causal relationship was not demonstrated.91 Subsequently, it was reported that MSCs differentiate into nurse-like cells that promote adhesion of lymphocytes to the synovium.92 Indeed, in patients with RA, but not healthy controls, bone marrow MSC-generating capacity is markedly reduced.93 Whether this is due to systemic TNF-alpha suppression of bone marrow,94 or exhaustion of MSC precursors by heightened demand is not known. However, there are suggestions of the latter based on observations of shorter telomeres in MSC derived from RA patients.93 The concept of MSC contributing to pathology was demonstrated in the CIA model by Djouad et al.,95 who reported that administration of MSC resulted in upregulation of Th1 immunity as determined by increased interferon production. and worsening of symptoms.95 The investigators attributed this to their observations that TNF-alpha abrogates immune regulatory activities of MSC. This study, however, was contradicted by several more recent studies in which inhibition of arthritogenesis, or even regression of disease was observed. Mao et al.,96 demonstrated that administration of rat MSC intravenously into DBA mice with full-blown CIA resulted in regression of disease, which was correlated with decreased production of TNF-alpha and IL-17.96 Gonzalez et al.,97 administered ex vivo-expanded human adipose-derived MSCs into the animals using the same model. Inhibition of disease progression was observed, which correlated with increased Treg numbers that were specific for CII. This study supports the previous principle discussed that an antigen-nonspecific tolerizing event may contribute to development of antigen-specific suppression.97 In addition to immune modulation, it is possible that cartilage tissue generated de novo from MSCs possesses a decreased level of immunogenicity.98 The overall anti-inflammatory/immune modulatory effects of MSCs have been demonstrated in a variety of settings, including in the mouse model of multiple sclerosis,99,100 transplant rejection,101 diabetes,102 SLE,103 and autoimmune enteropathy.104
Due to the systemic nature of RA, MSC have been primarily administered intravenously to treat this condition. Numerous studies have attested to the safety of single and multiple administration of these cells,105 with clinical trials demonstrating efficacy signals in a broad range of inflammatory conditions from heart failure,106 to stroke,107 to liver failure,108 and multiple sclerosis.109 Despite promising trials, currently the only MSC to have received marketing approval in the USA has been Ryoncil (remestemcel-L).110
Umbilical cord MSC (UC-MSC)
The original use of MSC in RA was a 2012 study that involved four patients with treatment-refractory disease. Supporting the efficacy of UC-MSC therapy, three of the patients had reduction in erythrocyte sedimentation rate (ESR) (a sensitive but non-specific measure of inflammation), DAS-28, and pain VAS score at 1 and 6 months after infusion. Two of the three responding patients had a European League against Rheumatism (EULAR) moderate response at 6 months but experienced a relapse at 7 and 23 months, respectively. The treatments did not cause any adverse effects though.111 These promising initial results led to expanded studies.
A subsequent study reported in 2013 treated 172 patients with active RA who had inadequate responses to standard of care. The patients were separated into Group 1 who received DMARDs plus culture media or Group 2 (no need to use grouping) who received UC-MSC 4×107 cells per timepoint via intravenous injection. No serious adverse effects were observed during or after infusion. The serum levels of tumor necrosis factor-alpha and interleukin-6 decreased and the percentage of Tregs in peripheral blood was increased after the first treatment. Significant disease remission was reported according to the American College of Rheumatology improvement criteria, the 28-joint disease activity score (DAS28), and the Health Assessment Questionnaire. The therapeutic effects were maintained for 3-6 months without continuous administration, correlating with the increased percentage of regulatory T cells of peripheral blood. No benefits were observed in control cohort that received DMARDS plus medium without UC-MSCs.112
Wang et al.,113 in 2016 used UC-MSC to treat juvenile idiopathic arthritis (JIA). Ten patients were treated with UC-MSCs and received a second infusion three months later. Synovial white blood cell count, ESR, TNF-alpha, IL-6 and CRP were significantly decreased while Tregs were increased, with these changes being maintained 6 months after treatment. These results suggest that UC-MSCs can reduce inflammatory cytokines, augment immune tolerance, and effectively alleviate the symptoms and they might provide a safe and novel approach for JIA treatment.113
In 2018, Park et al.,114 performed the CURE-iv trial, which was a phase I, uncontrolled, open-label trial for RA patients with moderate disease activity despite treatment with methotrexate. The patients received a single intravenous infusion of 2.5×107, 5×107, or 1×108 hUCB-MSCs for 30 minutes, three patients in each cluster, with an increment of cell numbers when there was no dose-limited adverse event. There was no major toxicity in all clusters up to 4 weeks after the infusion. Significant improvements in ESR and DAS28 were observed at 4 weeks, as well as reduction of inflammatory cytokines IL-1β, IL-6, IL-8, and TNF-α at 24 hours were observed in the cluster infused with 1×108 MSCs.114
In a study in 2019, four RA patients aged 18-64 years were recruited in the study. During the treatment, patients were treated with 40 mL UC-MSC suspension product (2 ×107 cells/20 mL) via intravenous injection immediately after the infusion of 100 mL saline. One year and 3 years after UC-MSC cells treatment, the routine, liver and kidney function and immunoglobulin examination showed no abnormalities, which were all in the normal range. The ESR, CRP, RF (Rheumatoid Factor) of 1 year and 3 years after treatment and anti-CCP of 3 years after treatment were detected to be lower than that of pretreatment, which showed significant change (p<0.05). Health index and joint function index (DAS28) decreased 1 year and 3 years after treatment.115
Adipose tissue MSC
In a 2016 clinical trial, allogeneic adipose derived MSC were administered to patients with active refractory RA (failure to at least two biologicals) were randomized to receive three intravenous infusions of allogeneic adipose MSC product called “Cx611”. Fifty-three patients received either a placebo or a treatment at doses of 1 million/kg, 2 million/kg, or 4 million/kg on days 1, 8, and 15, and were monitored for 24 weeks. Of those, 20 patients got 1 million/kg and had a 45% improvement rate at 1 month and 25% at 3 months; 20 patients got 2 million/kg and showed a 20% improvement rate at 1 month and 15% at 3 months; 6 patients received 4 million/kg and had a 33% improvement rate at 1 month and 17% at 3 months; and 7 patients received a placebo, with a 29% improvement rate at 1 month and 0% at 3 months.116
A 2022 trial reported the use of single, intravenous infusion of autologous, adipose-derived MSC in 15 RA patients. ACR66/68 scores for both S/TJC showed significant improvements with large effect sizes (ES) at week 52 vs baseline (p <0.01, ES = 0.83 and p <0.001, ES = 0.93 respectively). A difference in CRP levels with a small effect size was observed at week 4 (p =0.229, ES = 0.33) with further improvement at week 52 (p =0.183, ES = 0.37).117
Bone marrow MSC
In 2018, an Iranian study was to evaluate the effects of intravenous administration of autologous bone marrow-derived MSCs in nine refractory RA patients who received a single intravenous dose of 1×106 autologous bone marrow-derived MSCs/kg. A significant decreasing trend in Th17 percentage and geometric mean fluorescence intensity for IL-17A following injection of MSCs at 12 months compared to time point zero was observed. Furthermore, a significant increase in regulatory T cell percentage was seen at the end of the first month after the intervention. DAS28-ESR decreased significantly at 1 and 12 months after MSC therapy. VAS score showed a significant decreasing trend during the follow-up periods.118
In another juvenile arthritis study, six patients who failed biologicals received 2 million/kg intravenous infusions of allogeneic bone-marrow derived MSC. No acute infusion reactions were observed and a lower post-treatment than pre-treatment incidence in AEs was found. Statistically significant decreases were found 8 weeks after one MSC infusion in VAS well-being (75-56), the JADAS-71 (24.5-11.0) and the cJADAS10 (18.0-10.6).119
In 2020, a clinical trial used a single dose of 1×106 per kg autologous bone marrow MSCs in 13 patients suffering from refractory RA. The results showed that the gene expression of forkhead box P3 (FOXP3) in peripheral blood mononuclear cells (PBMCs) considerably increased at month 12. Additionally, they found a substantial increasing trend in the culture supernatant levels of IL-10 and transforming growth factor-beta 1 (TGF-β1) in PBMCs from the beginning of the intervention up to the end. These data reflect the sufficient immunoregulatory effect of autologous BM-MSCs on regulatory T cells in patients suffering from refractory RA.120
A 2024 study investigated the efficacy of 3 monthly intravenous infusions of allogeneic bone marrow-derived clonal mesenchymal stromal cells (BM-cMSCs) in six refractory RA patients. BM-cMSC infusions were well tolerated, with no SAEs reported. VAS scores improved in three patients, with two achieving sustained pain relief and quality-of-life enhancement. Four patients met ACR20 at week 16, while SDAI and CDAI scores indicated disease activity reduction in three patients. IL-10 increased in five patients, while pro-inflammatory markers TNF-α and IL-17 decreased in the same individuals.121
The above-mentioned clinical trials demonstrate the overall safety of administration of mesenchymal stem cells; however, efficacy is a matter of debate. One way to increase efficacy is the utilization of more immature types of stem cells, or “tailor-made” stem cells. Unfortunately, in many situations, the issue of donor-to-donor variability remains substantial. One way of overcoming this is the generation of MSC from pluripotent sources, especially if these pluripotent sources are personalized.
Immorta Bio Inc. has previously reported the ability of generating self-renewing, immortal pluripotent stem cells (Personalized Regenerative Cells (PRC)) from peripheral blood by application of proprietary dedifferentiation technology. Due to their ability for self-renewal, PRC can be genetically modified to express one or more autoantigens. Preliminary data shows successful differentiation of PRC into mesenchymal stem cells, which we term “pMSC”. By varying culture conditions, pMSC corresponding to various biological ages have been produced. Preliminary data shows superior reduction of RA score in the CIA model as compared to standard MSC types. Given the tolerogenic potential of these cells, work is being performed to generate autoantigen expressing autologous MSC, which we believe will be true facilitators of immunological tolerance induction.
DBA/1 LacJ female mice (ten per group), seven-ten weeks of age, were intradermally immunized (Day 0) at the base of the tail with 200 μg of bovine type II collagen (CII) (Sigma-Aldrich, St. Louis, MO, USA) with complete Freund's adjuvant (CFA) (Sigma). On Day 21 after priming, the mice received an intraperitoneal booster injection with 200 μg. Mice were examined visually three times a week for the appearance of arthritis in the peripheral joints, and an arthritis score index for disease severity was given as follows: 0 - no evidence of erythema and swelling; 1 -erythema and mild swelling confined to the mid-foot (tarsals) or ankle joint; 2 -erythema and mild swelling extending from the ankle to the mid-foot; 3 - erythema and moderate swelling extending from the ankle to the metatarsal joints; 4 -erythema and severe swelling encompassing the ankle, foot, and digits. The maximum possible score per mouse was 16. The severity of arthritis was also determined by the quantification of the paw swelling measured with a dial gauge caliper. Scoring was done by two independent observers, without knowledge of the experimental and control groups. Mesenchymal stem cells were administered two times a week for two weeks after the last booster shot. Mesenchymal stem cells were administered intravenously at a concentration of 500,000 cells per mouse. Control consisted of dermal fibroblasts, bone marrow MSC and umbilical cord MSC, which were obtained from ATCC. pMSC were produced from expanded PRC (Figure 1).
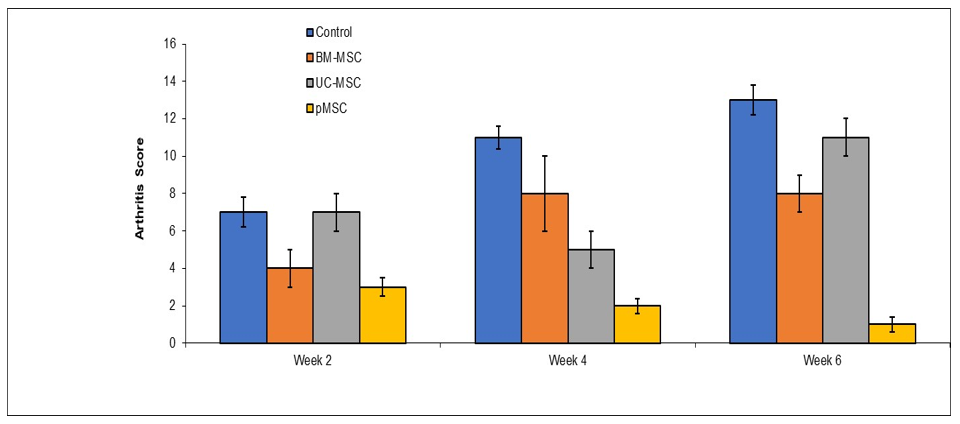
Figure 1 Efficacy of proprietary pluripotent-derived mesenchymal stem cells (pMSCs) in collagen-induced arthritis (CIA) model.
DBA/1 LacJ mice with collagen-induced arthritis (CIA) received intravenous infusions of different MSC preparations twice weekly for two weeks following disease onset. Disease severity was assessed by clinical arthritis scoring (0–4 per paw; maximum score 16 per mouse) and paw thickness measurements. Treatment with Immorta Bio’s proprietary pMSCs resulted in significantly greater reduction in arthritis scores and paw swelling compared to bone marrow-derived MSCs, umbilical cord MSCs, and dermal fibroblast controls.
Mesenchymal stem cells (MSCs) have emerged as promising candidates for the treatment of rheumatoid arthritis (RA) by addressing both inflammatory and degenerative aspects of the disease. Preclinical and clinical data demonstrate that MSCs can reduce inflammation, promote regulatory immune responses, and potentially contribute to tissue regeneration. However, variability in donor-derived MSC performance remains a key limitation. Our preliminary findings using proprietary pluripotent-derived MSCs (pMSCs) suggest enhanced therapeutic potential, particularly in inducing antigen-specific tolerance. Given the tolerogenic potential of these cells, work is being performed to generate autoantigen expressing autologous MSC which we believe will be true facilitators of immunological tolerance induction.
These results support further development of pMSC-based therapies as a next-generation, targeted, and personalized treatment strategy for RA.
None.
The authors declare that there are no conflicts of interest.

©2025 Ramos, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.