Journal of
eISSN: 2475-5540


Review Article Volume 10 Issue 1
1Biotechnology Department, California State University, USA
2Department of Bioengineering, University of California, USA
Correspondence: Dr. Bill Tawil, Department of Bioengineering, UCLA School of Engineering, 420 Westwood Plaza,Room 5121, Engineering V. P.O. Box: 951600, Los Angeles, CA 90095-1600, USA
Received: May 26, 2025 | Published: June 25, 2025
Citation: Norma D, Bill T. From missed mutations to liquid clues: a new era of EGFR diagnostics in NSCLC. J Stem Cell Res Ther. 2025;10(1):116-124. DOI: 10.15406/jsrt.2025.10.00193
Epidermal growth factor receptor (EGFR) is a key driver in the pathogenesis of non-small cell lung cancer (NSCLC) and colorectal cancer (CRC), making it a central target in precision oncology. This review examines the clinical utility of EGFR-targeted therapies, including tyrosine kinase inhibitors (TKIs) and monoclonal antibodies (mAbs), while addressing the mechanisms of therapeutic resistance that limit long-term efficacy. Emphasis is placed on the diagnostic landscape, from traditional PCR-based assays and tissue biopsies to advanced tools such as liquid biopsy, droplet digital PCR (ddPCR), and next-generation sequencing (NGS). The review also explores emerging platforms, including nanotechnology and CRISPR-based diagnostics, for their potential to enhance mutation detection accuracy and real-time monitoring. By evaluating current limitations, detection gaps, and integration challenges, this paper underscores the urgent need for multi-modal, high-sensitivity approaches to improve clinical decision-making and optimize outcomes in EGFR-driven malignancies.
Keywords: colorectal cancer, tyrosine kinase inhibitors, liquid biopsy, T790M, mutation detection, personalized therapy, next-generation sequencing, drug resistance, immunohistochemistry, CRISPR diagnostics
EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; CRC, colorectal cancer; TKI, tyrosine kinase inhibitor; mAb, monoclonal antibody; PCR, polymerase chain reaction; ctDNA, circulating tumor DNA; cfDNA, cell-free DNA; ddPCR, droplet digital PCR; ARMS-PCR, amplification refractory mutation system PCR; NGS, next-generation sequencing; IHC, immunohistochemistry; FACS, fluorescence-activated cell sorting; PET, positron emission tomography; PFS, progression-free survival; OS, overall survival; gRNA, guide RNA; dCas9, deactivated Cas9; ESDR, entropy-driven strand displacement reaction; CTC, circulating tumor cell; IPASS, IRESSA Pan-Asia Study; LCMC, lung cancer mutation consortium; CAGR, compound annual growth rate
Epidermal growth factor receptor (EGFR) is a key molecular target in precision oncology, particularly in non-small cell lung cancer (NSCLC) and colorectal cancer (CRC), where it plays a pivotal role in tumor growth, survival, and metastasis.1,2 Advances in targeted therapies, including tyrosine kinase inhibitors (TKIs) and monoclonal antibodies (mAbs), have significantly improved outcomes for patients with EGFR-mutant tumors. However, the success of these therapies is heavily dependent on accurate and timely mutation detection.3-5 Diagnostic platforms such as PCR, next-generation sequencing (NGS), and liquid biopsy have transformed EGFR testing, yet they still face challenges in sensitivity, specificity, and access. As resistance mutations—most notably T790M- continue to emerge, there is an urgent need for innovative and integrated detection strategies (Figure 1).1,2
Purpose
This review explores the clinical significance and therapeutic potential of EGFR-targeted treatments in EGFR-driven cancers, focusing particularly on NSCLC and CRC. Emphasis is placed on understanding the mechanisms of action and clinical applications of EGFR inhibitors- both TKIs and mAbs- along with current and emerging diagnostic technologies such as PCR, ddPCR, NGS, IHC, and CRISPR-based detection systems. The review also highlights resistance mechanisms, identifies diagnostic limitations, and presents opportunities for innovation in personalized medicine.
The epidermal growth factor receptor (EGFR) is a transmembrane receptor tyrosine kinase that plays a critical role in regulating cell proliferation, differentiation, migration, and survival. It is part of the ErbB receptor family, which includes EGFR (ErbB1), ErbB2 (HER2), ErbB3, and ErbB4, and becomes activated upon ligand binding, dimerization, and autophosphorylation of its intracellular tyrosine residues.2
Activation of EGFR triggers several key downstream signaling pathways, including the Ras-Raf-MEK-ERK (MAPK) cascade, the PI3K-AKT-mTOR pathway, and the PLCγ-PKC pathway, all of which contribute to oncogenesis when dysregulated. In NSCLC, activating mutations in the EGFR gene- most commonly deletions in exon 19 and the L858R point mutation in exon 21-lead to constitutive receptor activation, promoting uncontrolled tumor cell proliferation and survival (Figure 1 above).1,2
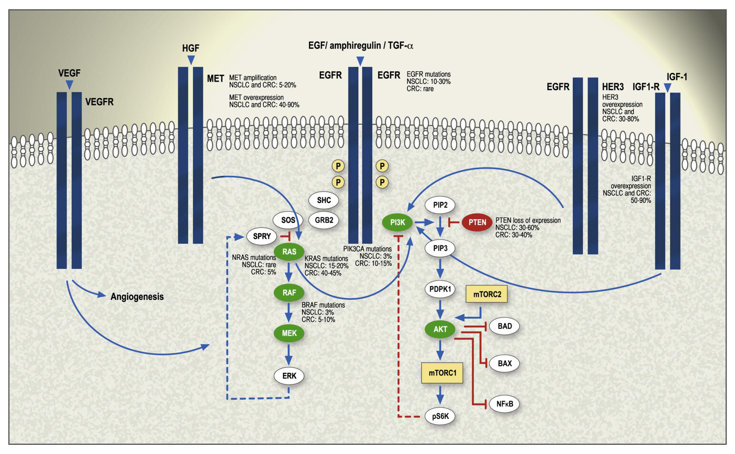
Figure 1 Structure and function of EGFR in cancer signaling.
Schematic representation of the EGFR receptor showing dimerization and activation of downstream pathways (RAS/MAPK, PI3K/AKT, and PLCγ/PKC) that regulate cell proliferation, survival, and differentiation.
EGFR mutations occur in approximately 10–15% of NSCLC cases in Western populations and up to 50% in Asian populations, with a higher prevalence among non-smokers and those with adenocarcinoma histology.1 These mutations confer sensitivity to EGFR tyrosine kinase inhibitors (TKIs), making EGFR one of the most actionable targets in lung cancer therapy.6,7
However, therapeutic response is often followed by resistance. The T790M mutation in exon 20 is the most common mechanism of acquired resistance, found in about half of patients who relapse after first- or second-generation TKI treatment. This mutation increases the affinity of EGFR for ATP, reducing the effectiveness of reversible TKIs like erlotinib and gefitinib. Other mechanisms of resistance include MET amplification, HER2 overexpression, histologic transformation, and bypass signaling through pathways such as IGF-1R and AXL.1
To address these challenges, second- and third-generation TKIs, such as afatinib, dacomitinib, and osimertinib, have been developed to target a broader spectrum of mutations and overcome resistance.1,8 Combination therapies involving EGFR inhibitors with agents targeting VEGF (e.g., bevacizumab) or downstream effectors like mTOR and PI3K are also under investigation, although results have been mixed in clinical trials.1
EGFR’s role extends beyond NSCLC. Aberrant EGFR signaling is also implicated in other malignancies, including colorectal cancer and head and neck squamous cell carcinoma, as well as non-cancerous conditions such as psoriasis and neurodegenerative diseases like Alzheimer’s disease.2
Overall, EGFR functions as a central driver of oncogenic signaling in NSCLC, and its biology underpins the rationale for targeted therapy, ongoing biomarker development, and precision medicine approaches in lung cancer management.1,2
Accurate identification of EGFR mutations is central to treatment planning in NSCLC. Multiple diagnostic techniques have been developed to address this need, each with unique advantages and limitations. These include tissue-based genotyping, liquid biopsy, polymerase chain reaction (PCR)-based assays, next-generation sequencing (NGS), immunohistochemistry (IHC), and emerging platforms like CRISPR and nanotechnology.4,5,9-14
Tissue-based testing
Tissue biopsy remains the gold standard for EGFR mutation analysis in non-small cell lung cancer (NSCLC), offering direct visualization of tumor cells, detailed histopathologic classification, and high-quality molecular profiling when sufficient tumor content is available.12 Compared to plasma-based testing, tissue analysis generally yields better DNA quality and quantity, enabling more accurate and sensitive mutation detection, especially for rare or low-frequency variants.12,15
In a comparative study by Kang et al., tissue-based testing demonstrated superior performance in identifying the T790M resistance mutation, detecting it in 34.1% of tissue samples compared to 18.6% in plasma.12 When both tissue and plasma methods were combined, the detection rate improved to 56.7%, underscoring the complementary role of liquid biopsy while reaffirming the diagnostic strength of tissue-based analysis in patients progressing on first- or second-generation tyrosine kinase inhibitors (TKIs) (Figure 2).12 Clinically, identifying a T790M mutation through tissue or liquid biopsy allows clinicians to switch patients to third-generation TKIs such as osimertinib. If T790M is not found, patients are typically transitioned to chemotherapy or alternative treatments.16
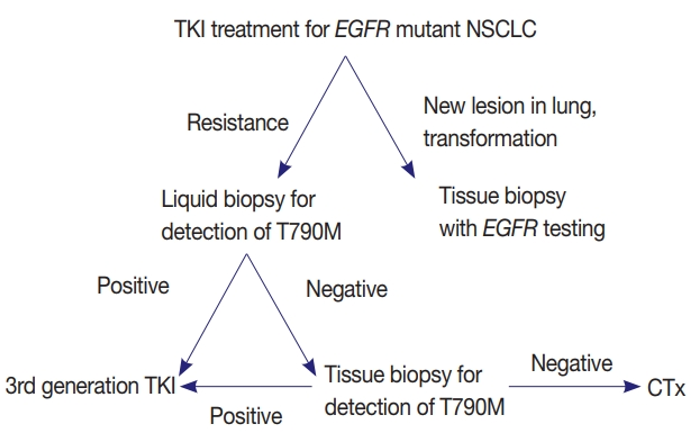
Figure 2 Clinical utility of liquid biopsy in EGFR-Mutant NSCLC.
Diagram illustrating how liquid biopsy enables real-time monitoring of EGFR mutations like T790M, allowing dynamic treatment adjustment with targeted therapies such as osimertinib.
Lee et al.,15 expanded on this by comparing ctDNA and tumor DNA (tDNA) testing in a large cohort. To ensure diagnostic clarity, 61 cases confirmed as EGFR wild-type by both ctDNA and tDNA methods were excluded. The remaining 421 cases, drawn from 368 patients, showed that tissue-based analysis revealed a broader spectrum of EGFR mutation types than plasma testing alone. This highlights the added value of integrating both testing modalities for comprehensive mutation profiling (Figure 3).15
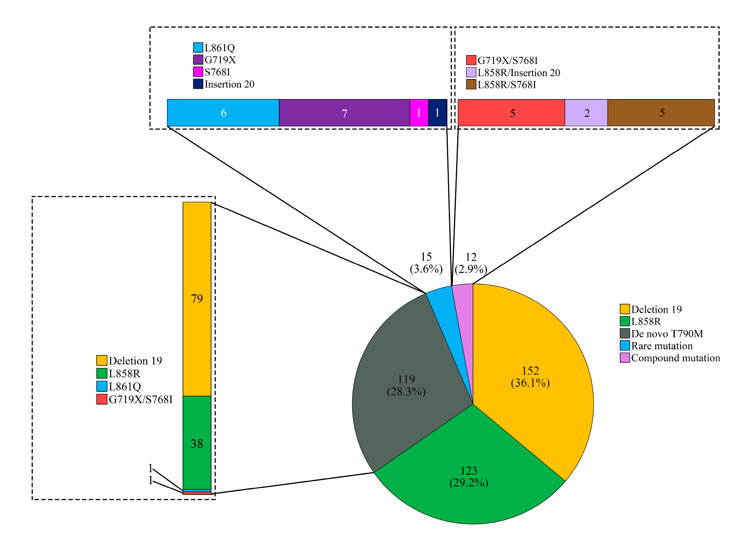
Figure 3 T790M detection rates: tissue vs. plasma testing.
Graph comparing detection rates of the T790M mutation in tissue-based, plasma-based, and combined methods in NSCLC patients.
Given these strengths, the Korean Cardiopulmonary Pathology Study Group recommends tissue biopsy as the preferred diagnostic approach for EGFR mutation testing when feasible. They emphasize that tissue analysis provides greater confidence in negative results and allows additional molecular assessments such as ALK or ROS1 testing to be performed on the same specimen.16
Altogether, tissue biopsy continues to play a central role in the molecular characterization of NSCLC. It remains the most reliable method for detecting both common and resistance-associated EGFR mutations and should be prioritized whenever patient condition and tumor accessibility allow.12,15,16
Liquid biopsy (Plasma-based testing)
Liquid biopsy analyzes circulating tumor DNA (ctDNA) in plasma and provides a non-invasive, repeatable alternative to tissue biopsy for EGFR mutation detection.3,17 This approach is especially useful in patients with advanced NSCLC, where repeated tissue sampling is not feasible or when tumor specimens are inadequate for comprehensive molecular analysis.14 Plasma-based genotyping enables real-time monitoring of tumor evolution, facilitates the early detection of resistance mutations like T790M, and supports timely adjustments to targeted therapies (Figure 4a).4,5,12,18
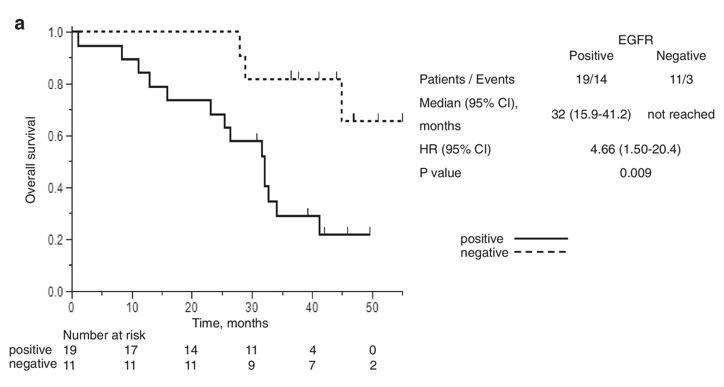
Figure 4a Prognostic value of liquid biopsy in EGFR-mutant NSCLC.
Kaplan-Meier curve comparing progression-free survival (PFS) and overall survival (OS) in patients diagnosed through tissue biopsy vs. liquid biopsy.
In a prospective study, Sacher et al. validated the clinical utility of plasma-based EGFR testing using digital PCR. The method demonstrated high sensitivity and a median turnaround time of only three days-substantially faster than traditional tissue-based workflows.17 This accelerated process enabled earlier therapeutic interventions and helped reduce treatment delays caused by biopsy scheduling or sample processing limitations.
Comparative data from Kang et al. revealed that T790M mutations were identified in 18.6% of plasma samples versus 34.1% of tissue samples, with the combined approach yielding a 56.7% detection rate—underscoring the complementary value of liquid biopsy in mutation profiling.12 This integration is particularly impactful in patients with suspected acquired resistance, where time-sensitive treatment decisions must be made.
Ke et al.,18 further explored the association between resistance mutations and specific EGFR subtypes. Their study found that the T790M mutation appeared more frequently in patients with exon 19 deletions than those with L858R mutations. This molecular distinction may help explain the improved survival outcomes commonly observed in patients with exon 19 deletions, reinforcing the dual prognostic and diagnostic importance of plasma-based testing (Figure 4b).
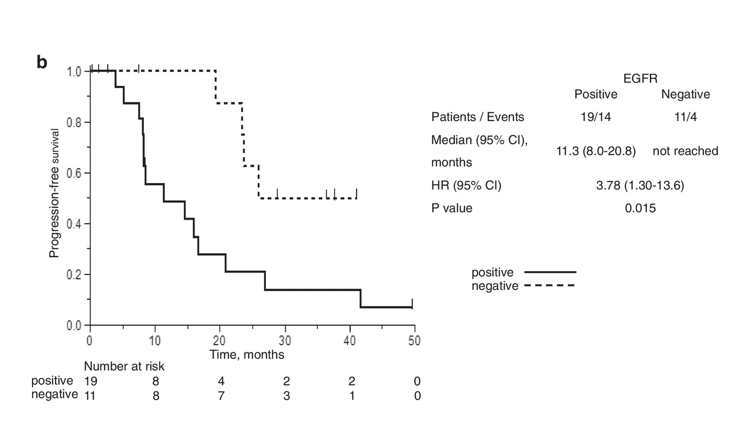
Figure 4b Monitoring tumor evolution using ctDNA.
Visualization of how ctDNA levels change over time with therapy, providing early indicators of resistance mutations and guiding treatment decisions.
Normanno et al.,4 emphasized that although ctDNA assays are promising, their performance depends on several factors, including ctDNA abundance, sample handling, and the sensitivity of the chosen assay platform.14 Casula et al.,5 compared three liquid biopsy technologies—Therascreen®, Idylla™, and NGS- and confirmed that detection sensitivity varies, particularly for mutations like T790M and L858R.5 These findings highlight the importance of selecting the most appropriate platform based on clinical context and patient-specific variables.
Overall, liquid biopsy serves as a powerful adjunct to tissue-based testing, offering rapid turnaround, minimal invasiveness, and dynamic insights into tumor evolution. It is especially beneficial for detecting resistance mutations, monitoring treatment response, and guiding real-time therapy decisions. Moreover, in patients who are not candidates for repeat biopsy, liquid biopsy provides a clinically valuable alternative for early resistance detection, prognosis assessment, and individualized treatment planning (Figures 4a & 4b above).4,5,12,14,17-19
PCR-based technique
PCR-based assays, including Scorpion-ARMS and the cobas® EGFR Mutation Test v2, are widely used in clinical practice due to their simplicity, rapid turnaround time, and compatibility with formalin-fixed, paraffin-embedded samples. These techniques are effective for detecting common EGFR mutations, particularly exon 19 deletions and the L858R point mutation in exon 21, and are frequently used as frontline assays in diagnostic workflows. However, their utility is limited when it comes to identifying rare, low-frequency, or complex mutations. The sensitivity of these assays is constrained by their limit of detection (LOD), often requiring a variant allele frequency above 1% to 5% to produce a reliable signal.13
In a study by Matsubara et al.,13 several EGFR mutations in NSCLC samples went undetected by conventional PCR-based assays, despite being confirmed through more sensitive techniques. Notably, compound mutations-such as L858R combined with G863D- were frequently missed by the cobas® test due to design limitations that focus only on the most common variants. These undetected mutations can have clinical relevance, as patients harboring them may still benefit from EGFR tyrosine kinase inhibitor (TKI) therapy but are excluded from treatment due to false-negative results. The study highlighted that reliance on PCR-based methods alone could lead to misclassification and under-treatment of a subset of NSCLC patients, particularly those with rare or compound EGFR alterations.13
Given these limitations, Matsubara et al. advocated for the use of more comprehensive diagnostic platforms- such as NGS- in cases where PCR results are negative but clinical suspicion remains high or when compound mutations are suspected.13 This approach ensures that actionable mutations are not overlooked, ultimately supporting more accurate treatment decisions and better patient outcomes.
Next-generation sequencing (NGS)
Next-generation sequencing (NGS) offers comprehensive genomic profiling and has become an essential tool in the molecular characterization of non-small cell lung cancer (NSCLC).4,14 Unlike targeted PCR-based assays, NGS allows for the simultaneous analysis of multiple exons and genes, enabling the detection of both common EGFR mutations-such as exon 19 deletions and the L858R point mutation-and rarer variants including exon 20 insertions, G719X, and L861Q. NGS is also capable of identifying co-occurring mutations, which may impact response to targeted therapies or suggest alternative therapeutic approaches.11
In a large retrospective study of 4,467 NSCLC cases, He et al. demonstrated that NGS significantly outperformed ARMS-PCR in detecting EGFR mutations, particularly low-frequency and rare variants. NGS showed higher sensitivity for less common mutations such as G719X and L861Q and was more effective in detecting complex mutation patterns, including compound mutations and mutations present at low allele frequencies. The study also highlighted that NGS uncovered additional clinically relevant mutations not detected by ARMS-PCR, supporting its utility for comprehensive molecular profiling in advanced or treatment-resistant cases.14
Normanno et al.,4 also emphasized the added value of NGS in scenarios where ctDNA analysis is required, especially when used on plasma samples from patients with advanced disease. NGS-based ctDNA testing provides greater coverage than single-gene assays and is particularly useful when tissue samples are unavailable or inadequate. However, they noted that sensitivity can still be influenced by ctDNA quantity and quality, underscoring the importance of assay optimization and sample handling.14
Together, these findings establish NGS as a superior method for broad and sensitive detection of EGFR mutations, particularly when rare, co-occurring, or subclonal mutations are suspected. While more resource-intensive than PCR-based approaches, its expanded detection capabilities make it a valuable tool in guiding personalized treatment strategies in NSCLC.4,14
Immunohistochemistry (IHC)
Immunohistochemistry (IHC) using mutation-specific antibodies provides a cost-effective and rapid alternative for screening EGFR mutations in lung cancer. This method employs antibodies that specifically recognize common activating mutations, such as L858R and exon 19 deletions (particularly E746-A750), directly in formalin-fixed, paraffin-embedded tissue sections. The L858R-specific antibody demonstrated reliable reactivity with all L858R-positive tumors and even with some nearby point mutations, suggesting a high degree of specificity in that context. In contrast, the antibody targeting exon 19 deletions showed limited reactivity, detecting only a subset of deletion variants, thereby highlighting its restricted mutation coverage.10
In a validation study involving 238 lung cancer cases, IHC using these mutation-specific antibodies achieved an overall sensitivity of up to 92%, indicating strong potential as a screening tool. However, specificity was considerably lower—approximately 37%—due to the occurrence of false positives and variability in staining patterns, particularly in wild-type tumors or those harboring less common EGFR alterations. Moreover, significant intratumoral heterogeneity was observed, with 46% of tumors exhibiting inconsistent staining across different tissue cores in the same tumor sample. This heterogeneity raises concerns about the reliability of IHC in small biopsies or limited tissue samples, where sampling bias could lead to misinterpretation of EGFR mutation status.10
Overall, while IHC with mutation-specific antibodies offers practical advantages in speed and cost, its diagnostic accuracy is limited by narrow mutation coverage, low specificity, and staining inconsistency. As such, it is best used as a supplementary tool rather than a standalone method for EGFR mutation detection.10
Balancing diagnostic strengths through integrated testing
Each EGFR mutation detection method offers unique strengths and limitations, and using them in combination can help address individual weaknesses and improve diagnostic accuracy.20 For example, next-generation sequencing (NGS) provides a comprehensive mutation profile, including rare and co-occurring alterations, but may miss low-frequency mutations due to detection limits or sample quality.4,9,14 In contrast, liquid biopsy offers a less invasive and more easily repeatable option, but it may fail to capture mutations present in solid tumor cells, particularly in patients with low circulating tumor DNA (ctDNA) levels or early-stage disease.4,12,17,20
Multiplex testing strategies- where multiple platforms such as PCR, NGS, and liquid biopsy are used together- can increase sensitivity, reduce false negatives, and offer a more complete picture of the tumor's mutational landscape.14,20 Advanced PCR formats, such as droplet digital PCR (ddPCR), have improved sensitivity, detecting EGFR mutations at variant allele frequencies as low as 0.1%.4,21 ddPCR has demonstrated utility in both tissue and plasma samples and has been shown to outperform ARMS-PCR in detecting low-abundance mutations.18
Tissue biopsy remains the gold standard for initial mutation testing due to its high sensitivity and ability to provide histopathological context; however, it is invasive and sometimes not feasible due to tumor location or patient condition.10 Moreover, a single-site biopsy may not capture the full extent of intratumoral heterogeneity, which can result in missed subclonal mutations.3,20
Immunohistochemistry (IHC) can serve as a rapid, low-cost screening tool and is especially valuable in resource-limited settings, but it lacks the specificity and mutation coverage of molecular methods and should not be used as a standalone diagnostic approach.8,21 Although the adoption of NGS in clinical practice is expanding, it remains limited by high costs, long turnaround times, and the need for specialized infrastructure and data interpretation expertise.9,12 As testing technologies evolve, integrated strategies that leverage the strengths of each platform will be critical for accurate, timely, and personalized patient care.
Advancements in diagnostic technologies are reshaping the landscape of precision oncology, particularly in the detection of EGFR mutations in non-small cell lung cancer (NSCLC). Two rapidly developing frontiers- CRISPR-based diagnostics and nanotechnology- are showing strong potential to overcome the limitations of traditional mutation detection methods.
CRISPR-based diagnostics
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats)-based diagnostic tools leverage the sequence-specific recognition and cleavage properties of CRISPR-associated (Cas) proteins to detect genetic mutations with high precision and speed.7 Unlike conventional PCR or sequencing methods, CRISPR diagnostics do not require extensive thermal cycling or large-scale instrumentation, making them particularly attractive for rapid, point-of-care applications.
Diagnostic platforms such as DETECTR (DNA Endonuclease Targeted CRISPR Trans Reporter) and SHERLOCK (Specific High Sensitivity Enzymatic Reporter UnLOCKing) utilize the trans-cleavage activity of Cas12 and Cas13 enzymes, respectively, to generate detectable signals upon binding to a target DNA or RNA sequence (Figure 5).7,22 These platforms can identify EGFR mutations at very low concentrations—down to the attomolar level—making them suitable for detecting mutations in plasma-derived circulating tumor DNA (ctDNA).7
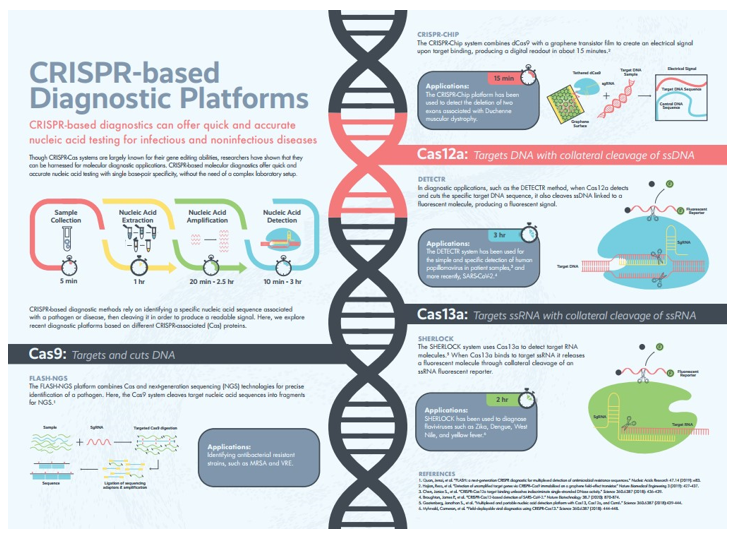
Figure 5 CRISPR-based diagnostics.
Though widely known for gene editing, CRISPR-Cas systems are also emerging as precise, rapid, and accessible platforms for nucleic acid detection with single base-pair resolution.
In addition to their sensitivity, CRISPR diagnostics are highly adaptable. Techniques like dCas9-based detection systems use catalytically inactive Cas9 proteins to bind, but not cleave, target sequences, enabling high-specificity mutation detection without disrupting genomic material. Other CRISPR approaches, such as FLASH (Finding Low Abundance Sequences by Hybridization), employ sequence-specific enrichment strategies to increase the likelihood of detecting rare mutations like T790M or exon 20 insertions. Though still in early clinical validation stages, CRISPR diagnostics have already shown utility in detecting cancer mutations, infectious pathogens (e.g., SARS-CoV-2), and even antibiotic resistance genes, underlining their broad applicability.7
Nanotechnology for single-cell analysis of CTCs
Nanotechnology is another transformative area in cancer diagnostics, particularly in the detection and analysis of circulating tumor cells (CTCs). These cells, shed from primary tumors into the bloodstream, carry important molecular information that reflects both the genetic profile and phenotypic heterogeneity of tumors. However, traditional CTC isolation methods lack sensitivity and specificity, especially in early-stage cancers or low-burden disease. Emerging nanotechnology platforms such as NanoVelcro chips and microfluidic devices have been developed to improve the isolation, capture, and molecular profiling of CTCs from patient blood samples. NanoVelcro technology employs nanoscale substrates coated with antibodies or other targeting ligands that selectively bind to surface markers expressed on tumor cells, significantly enhancing capture efficiency. Microfluidic platforms offer size- and deformability-based CTC separation and allow for real-time analysis of captured cells under flow conditions. These nanotechnology-enabled approaches facilitate single-cell analysis, allowing researchers to perform genomic and transcriptomic profiling at the level of individual CTCs. This is especially useful in understanding tumor heterogeneity and in detecting EGFR mutations that may be present in only a subset of the tumor population. Technologies like laser microdissection and dual-mode release systems further refine single-cell capture and analysis, ensuring minimal contamination and high-integrity molecular data. Together, these innovations allow for non-invasive, high-resolution, and real-time monitoring of tumor evolution, treatment response, and resistance development. Nanotechnology-based platforms are also particularly promising for early detection applications, as they can identify CTCs even in asymptomatic patients with very low tumor burdens-potentially enabling diagnosis before radiologic or symptomatic presentation.23
While both CRISPR-based diagnostics and nanotechnology platforms are still transitioning from bench to bedside, their potential to enhance sensitivity, reduce turnaround time, and enable real-time monitoring positions them as powerful complements to existing EGFR testing methods. Their eventual integration into clinical workflows could support more personalized and adaptive treatment strategies for NSCLC and other EGFR-driven cancers.
Clinical applications and significance
The clinical utility of EGFR mutation testing extends beyond its diagnostic function-it is central to personalized treatment planning, resistance monitoring, prognosis assessment, and real-time therapeutic adaptation in non-small cell lung cancer (NSCLC). As therapeutic options evolve, accurate and dynamic mutation profiling remains critical for improving clinical outcomes.
EGFR mutations as therapeutic targets
Mutations such as exon 19 deletions and L858R in exon 21 are well-established predictive biomarkers for response to EGFR tyrosine kinase inhibitors (TKIs). The IPASS trial demonstrated that gefitinib led to superior progression-free survival (PFS) in EGFR-mutant patients compared to chemotherapy (HR 0.48), with an objective response rate (ORR) of 71.2% versus 47.3% in the chemotherapy group.5 The EURTAC trial echoed these findings, showing erlotinib significantly improved PFS in mutation-positive individuals.24 These results emphasized the necessity of genotyping prior to initiating first-line therapy.
However, resistance inevitably emerges. The T790M gatekeeper mutation accounts for 50–60% of resistance cases following first- or second-generation TKI treatment.30 Osimertinib, a third-generation TKI, was developed to overcome this mutation. The AURA3 trial confirmed its efficacy in T790M-positive patients, where osimertinib significantly prolonged PFS compared to chemotherapy.6
Liquid biopsy in clinical decision-making
Liquid biopsy has transformed EGFR testing by enabling non-invasive, repeatable, and rapid assessment of tumor mutation status. Sacher et al.,15 reported that plasma genotyping using ddPCR achieved a turnaround time of three days and accurately detected actionable mutations in a large percentage of advanced NSCLC patients. Fujii et al.,17 found that EGFR mutations identified through liquid biopsy were correlated with worse PFS and OS in patients treated with afatinib, suggesting its utility extends beyond diagnosis into prognostic stratification.
ddPCR also outperformed ARMS-PCR in detecting low-frequency mutations in plasma, which is critical in patients with minimal tumor burden or difficult-to-biopsy lesions. However, platforms vary in sensitivity, and some, including ARMS-PCR, may miss rare mutations like exon 20 insertions, highlighting the need for multiplex approaches.19
Clinical trials supporting mutation-guided therapy
Clinical trials reinforce the value of EGFR testing in therapy selection. The TRIBUTE trial, which added erlotinib to chemotherapy without prior genotyping, failed to show survival benefit, underscoring the inefficacy of EGFR inhibitors in unselected populations.25 In contrast, the FASTACT-2 trial employed a sequential strategy of chemotherapy followed by erlotinib and showed improved PFS, especially in EGFR-mutant patients. Importantly, FASTACT-2 incorporated serial plasma genotyping, allowing for real-time mutation tracking. This enabled timely treatment adjustments in response to emerging resistance, a paradigm now central to managing advanced NSCLC.26
EGFR mutation monitoring and prognosis
Beyond guiding treatment, EGFR mutation patterns carry prognostic weight. Ke et al.,15 found that patients harboring both Del19 and T790M mutations had more favorable post-progression survival than those with L858R, pointing to differences in disease biology and resistance kinetics. Similarly, studies by the Lung Cancer Mutation Consortium (LCMC) and TRIBUTE trial showed that high EGFR expression in wild-type tumors is associated with poorer prognosis, reinforcing EGFR’s dual role as a therapeutic and prognostic biomarker.
Integration of testing methods for clinical accuracy
Both plasma and tissue testing contribute unique strengths. Tissue biopsies allow detailed histologic and molecular profiling, but they are invasive and sometimes not feasible due to tumor location or patient condition.10 Plasma-based methods offer accessibility and repeatability, but their sensitivity is limited in low-shedding tumors or early-stage disease.3
Kang et al.,10 demonstrated that tissue testing detected T790M in 34.1% of cases, while plasma testing identified it in 18.6%. The combined use of both methods raised the overall detection rate to 56.7%, highlighting the benefit of integrated strategies (Figure 5 above).10 Additionally, Casula et al.,4 reported 100% concordance between Idylla™ and Therascreen® platforms in detecting T790M, supporting the reliability of advanced plasma assays when used appropriately (Figure 6).4
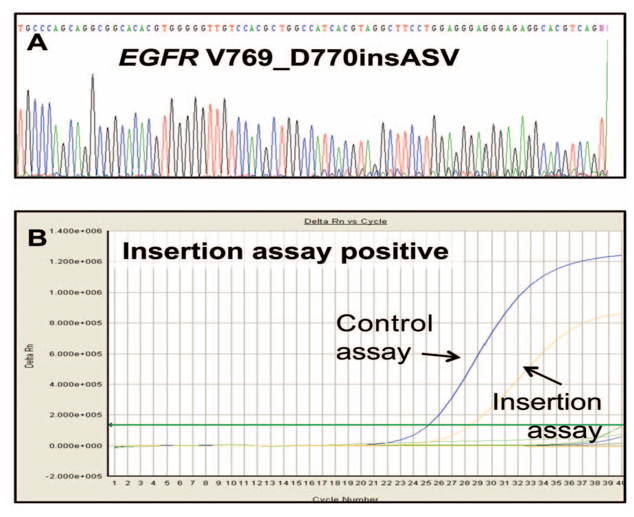
Figure 6 Comparison of direct sequencing and Therascreen EGFR mutation test.
An exon 20 insertion (V769_D770insASV) appears faint in direct sequencing due to low signal intensity, while the Therascreen assay provides a clearer, more definitive result.
Clinical utility of IHC and alternative platforms
Immunohistochemistry (IHC) using mutation-specific antibodies targeting exon 19 deletions and L858R offers a rapid and cost-effective option for EGFR mutation screening. In a cohort of 238 lung cancer samples, IHC demonstrated high sensitivity (up to 92%) but limited specificity (~37%), largely due to heterogeneous staining and false positives. Nearly half of the tumors showed intratumoral variation in staining patterns, raising concerns about sampling bias-particularly in small biopsies. Importantly, strong positive staining (score 3+) with mutation-specific antibodies was generally associated with increased EGFR gene copy number, a trend also observed with total EGFR antibodies (Figure 7). However, this correlation was not consistent-some cases with high EGFR expression lacked strong staining, while others showed uneven antibody reactivity, reflecting tumor heterogeneity and complicating interpretation. Given these limitations, IHC is best used as an adjunct in settings lacking access to molecular testing platforms. While useful for screening and detecting EGFR overexpression, IHC results should be confirmed by PCR or next-generation sequencing (NGS) for clinical decision-making.8
Despite considerable progress in EGFR-targeted therapies and mutation detection technologies, several persistent challenges continue to impede the full realization of personalized treatment for non-small cell lung cancer (NSCLC). One of the most critical issues is the limited sensitivity and specificity of widely used diagnostic assays. PCR-based platforms such as the cobas® EGFR Mutation Test v2 and Scorpion-ARMS, while efficient for detecting common alterations like exon 19 deletions and L858R, often miss rare or compound mutations, including clinically relevant combinations like L858R + G863D. These undetected variants may lead to false-negative results, causing patients to be inappropriately excluded from EGFR-TKI therapy, despite potential benefit.11
Intra-tumoral heterogeneity further complicates accurate detection. Tumors often consist of multiple clonal populations, and a single-site biopsy may fail to capture this complexity, particularly if resistance-associated mutations are confined to subclonal populations.3,20 This limitation not only affects initial diagnosis but also undermines ongoing therapeutic monitoring, as resistance mutations can emerge under selective pressure from targeted treatments.3
Liquid biopsy has emerged as a non-invasive alternative for mutation monitoring, offering the ability to assess ctDNA in real time and detect evolving mutations such as T790M.3,4 However, plasma-based testing is also limited by reduced sensitivity when ctDNA levels are low, which is common in patients with minimal tumor burden or early-stage disease. Additionally, the variability in ctDNA shedding among patients can lead to inconsistent detection outcomes, making liquid biopsy an incomplete replacement for tissue-based methods in some cases.10
Another significant constraint is sample quality. Studies indicate that mutation detection accuracy is directly affected by the tumor content ratio in biopsy specimens- samples with low tumor cellularity can compromise test performance, particularly for assays with higher limits of detection. This issue is exacerbated in small biopsies or fine-needle aspirations, where obtaining sufficient material for comprehensive analysis is often challenging.27
The detection of resistance mutations such as T790M and S768I also remains problematic due to their low variant allele frequency, requiring highly sensitive techniques to ensure accurate identification.28,29 Missed resistance mutations can delay appropriate treatment escalation and contribute to disease progression.
Advanced technologies like next-generation sequencing (NGS) and CRISPR-based diagnostics have emerged as promising solutions to these limitations. NGS offers broad mutation coverage and can detect rare, low-frequency, and co-occurring mutations with high sensitivity. However, barriers such as cost, limited availability, long turnaround times, and the need for advanced bioinformatics support continue to restrict widespread adoption in clinical settings.9,12 Similarly, CRISPR-based assays hold the potential for rapid and ultra-sensitive mutation detection, but these platforms are still in early stages of translation and are not yet widely implemented in routine diagnostics.7
Key limitations in current EGFR mutation detection strategies include:
Overcoming these challenges will require a multi-platform diagnostic approach that integrates tissue and plasma testing, employs highly sensitive technologies, and ensures access to comprehensive sequencing methods. In parallel, investments in infrastructure and clinician training are needed to support advanced molecular diagnostics and optimize their use in patient care. By addressing these gaps, the field can move closer to realizing the full potential of precision oncology-ensuring that every patient receives a timely and accurate diagnosis and access to the most effective, individualized treatment options.
The future of EGFR mutation detection lies in the development and clinical integration of more sensitive, rapid, and accessible diagnostic tools. Combining tissue- and plasma-based testing has already improved mutation detection rates, particularly for resistance mutations like T790M, and this dual approach is likely to become standard practice in NSCLC management.10,13
Emerging technologies offer significant promise. CRISPR-based diagnostics, including DETECTR and SHERLOCK platforms, provide highly specific and rapid detection of EGFR mutations at very low allele frequencies, offering potential applications in point-of-care settings.7 Their ability to combine sequence-specific targeting with signal amplification makes them well-suited for detecting low-abundance mutations in blood-based samples. Although still in early clinical adoption, these tools could eventually complement or replace traditional laboratory assays.
Artificial intelligence (AI) and machine learning are also gaining traction in the field. These technologies can analyze radiographic and histopathologic data to predict EGFR mutation status, offering a non-invasive adjunct to molecular testing.30 AI-driven models may one day triage patients for testing or treatment selection, streamlining workflows and reducing diagnostic delays.
Multiplexed testing strategies- leveraging PCR for rapid screening and NGS for comprehensive profiling are expected to define future diagnostic standards. This combination balances speed, depth, and cost while improving detection of co-occurring mutations that may influence therapeutic outcomes.12
The global EGFR testing market reflects these trends. In 2023, it was valued at $17.4 million, with Sanger sequencing holding the largest share due to its broad mutation detection capability. Although its projected annual growth is modest, the global EGFR mutation test market is expected to grow from $550 million in 2023 to $1.2 billion by 2032, driven by demand for precision medicine, especially in regions like North America and Europe.31,32
To meet clinical and market demands, future efforts must prioritize:
With continued innovation and cross-sector collaboration, EGFR mutation detection is positioned to become more efficient, predictive, and equitable- central to the next era of personalized oncology.
EGFR-targeted therapies have significantly reshaped the treatment landscape for non-small cell lung cancer (NSCLC) and other EGFR-driven malignancies. First- and second-generation tyrosine kinase inhibitors (TKIs), such as gefitinib and erlotinib, have demonstrated clear clinical benefits in patients harboring common EGFR mutations like exon 19 deletions and L858R.1,5,24 However, the effectiveness of these therapies hinges on accurate, timely, and comprehensive mutation detection. Standard PCR-based methods remain limited in their ability to detect rare, compound, or resistance-associated mutations- such as T790M and S768I- which can result in missed therapeutic opportunities and accelerate treatment resistance.11,28,29
To overcome these diagnostic limitations, more advanced platforms have been introduced. Next-generation sequencing (NGS) enables broad mutation profiling and is particularly effective for identifying low-frequency and complex mutations that are undetectable by PCR alone.3,11 Digital PCR formats like droplet digital PCR (ddPCR) offer higher sensitivity for known variants, including T790M, and have shown superior performance compared to ARMS-PCR in detecting mutations from both tissue and plasma samples.3,19 Liquid biopsy has further enhanced clinical flexibility by enabling non-invasive, repeatable testing that reflects tumor heterogeneity and real-time mutation evolution, making it a powerful tool for resistance monitoring and adaptive treatment planning.16,24–26 Meanwhile, emerging tools such as CRISPR-based diagnostics and nanotechnology-based CTC analysis are expanding the possibilities for ultra-sensitive, rapid, and point-of-care testing.7,23
Despite these innovations, barriers remain. NGS and CRISPR technologies, while promising, are often limited by cost, longer turnaround times, and the need for specialized infrastructure and data interpretation expertise.10,14,28 Liquid biopsy sensitivity is still suboptimal in low-shedding tumors, and tissue biopsies remain invasive and sometimes impractical due to tumor location or patient condition. These challenges highlight the need for an integrated, multi-platform diagnostic approach that leverages the strengths of each technology to maximize detection accuracy and clinical utility.12,18
Ultimately, enhancing EGFR mutation detection is not merely a technical goal-it is a cornerstone of precision oncology.33,34 Ensuring that every patient receives an accurate molecular diagnosis is essential for guiding targeted therapy, avoiding ineffective treatments, and improving outcomes. As the field advances, coordinated collaboration across research, diagnostics, regulatory bodies, and clinical practice will be critical to translating emerging technologies into accessible, real-world solutions. Only through such alignment can the full potential of EGFR-targeted therapy be realized in every stage and setting of lung cancer care.
There is no funding to report for this study.
Companies
1a. Roche Diagnostics: https://diagnostics.roche.com/us/en/home.html
1b. Quiagen: https://www.qiagen.com/us
1c. Biocartis: https://www.biocartis.com/en-US
Products
2a. cobas® EGFR Mutation Test v2: https://diagnostics.roche.com/us/en/products/lab/cobas-egfr-mutation-test-v2-ivd-rmd-4800-egfrv2-003.html
2b. therascreen KRAS RGQ PCR Kit:
2c. IdyllaTM Platform: https://www.qiagen.com/us/products/diagnostics-and-clinical-research/oncology/therascreen-solid-tumor/therascreen-kras-rgq-pcr-kit-us
Author has no acknowledgements to address.
The authors declare that there are no conflicts of interest.

©2025 Norma, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.