Journal of
eISSN: 2475-5540


Review Article Volume 10 Issue 1
Department of Bioengineering, University of California, USA
Correspondence: Dr. Bill Tawil, Department of Bioengineering, UCLA School of Engineering, 420 Westwood Plaza, Room 5121, and Engineering V. P.O. Box: 951600, Los Angeles, CA 90095-1600, USA
Received: June 26, 2025 | Published: July 15, 2025
Citation: Makena R, Bill T. Breast reconstruction in the modern era: biological insights, market advances, and emerging technologies . J Stem Cell Res Ther. 2025;10(1):131-140. DOI: 10.15406/jsrt.2025.10.00195
Breast reconstruction is a multidisciplinary field at the intersection of surgical oncology, plastic surgery, and tissue engineering, aiming to restore form, symmetry, and patient well-being following breast tissue removal. The field has evolved from relying solely on autologous tissue and silicone implants to exploring regenerative strategies involving stem cells, bioprinted scaffolds, and synthetic meshes that support integration and vascularization. This review aims to synthesize current advances in breast reconstruction technologies, discuss clinical adoption and market trends, and highlight how bioengineering innovations transform traditional reconstructive options. In addition to evaluating marketed and investigational products, this paper briefly considers how the biology of healthy and diseased breast tissue impacts reconstructive performance and how diagnostic delays influence reconstruction planning. By linking tissue-level pathophysiology to reconstructive outcomes, the paper underscores the need for precision-engineered solutions that adapt to diverse anatomical and clinical challenges.
Keywords: breast reconstruction, tissue engineering, regenerative medicine, autologous fat grafting, 3D bioprinting, adipose-derived stem cells, implant-based reconstruction, acellular dermal matrix, patient-reported outcomes
3D, three dimensional; ECM, extracellular matrix; ADM, acellular dermal matrix; MRI, magnetic resonance imaging; DTI, direct-to-implant; PCL, polycaprolactone; PLA, polylactic acid; PEGDA, polyethylene glycol diacrylate; ADSC, adipose-derived stem cell; SVF, stromal vascular fraction; NAC, nipple-areolar complex; CAL, cell-assisted lipotransfer; TA, tumor-associated macrophage; TAF, tumor-associated fibroblast; MDSC, myeloid-derived suppressor cell; PD-L1, programmed death-ligand 1; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; TLR, toll-like receptor; BRCA, breast cancer gene; DCIS, ductal carcinoma in situ; PROM, patient-reported outcome measure; BREAST-Q, breast reconstruction evaluation assessment scale tool -Questionnaire
Breast cancer is the most common malignancy among women globally, with over 2.3 million new cases reported annually, making surgical intervention a cornerstone of treatment.1-4 Surgical approaches vary significantly depending on tumor size, location, and patient preference, ranging from breast-conserving surgery (lumpectomy) to more extensive options such as skin-sparing, nipple-sparing, and modified radical mastectomy.5 Factors influencing surgical choice extend beyond clinical staging, including hereditary cancer syndromes (e.g., BRCA1/2), family history, imaging findings, and patient anxiety regarding recurrence.6 Some women elect prophylactic mastectomy even in the absence of a current cancer diagnosis to reduce risk, reflecting growing patient autonomy and awareness.7 Despite the increasing availability of reconstructive procedures, not all patients choose to undergo breast reconstruction.8 As of 2014, reconstruction uptake in the U.S. hovered around 43%, highlighting gaps in access, information, or personal inclination.8 Breast reconstruction is typically presented as an optional procedure, discussed between the patient and care team, and it may occur immediately after mastectomy (immediate reconstruction) or months to years later (delayed reconstruction), depending on treatment course and patient readiness.9
The physical and emotional toll of mastectomy can be profound, and breast reconstruction offers a restorative option that helps many patients regain a sense of femininity, normalcy, and self-confidence.10 Psychological benefits of reconstruction include reduced body image distress, improved sexual satisfaction, and a better overall quality of life, especially when patients feel their appearance has been successfully restored.11 Studies using validated outcome measures, such as the BREAST-Q, have demonstrated that both implant-based and autologous approaches yield high satisfaction scores. However, preferences vary based on individual priorities like recovery time, appearance, and complications.12 Over the past two decades, there has been a cultural and medical shift toward patient empowerment and shared decision-making in reconstruction planning, ensuring patients are aware of all options, including doing nothing.13 Increased awareness, improvements in reconstructive techniques, and insurance mandates requiring physicians to inform patients of reconstructive options have helped drive the rise in reconstruction rates.7 Nevertheless, disparities still exist: racial, economic, and geographic inequities continue to influence who receives reconstruction and what types are offered or accessible.14
To further improve breast reconstruction outcomes, recent research has examined how biological factors, such as the structure of healthy versus diseased tissue, affect scaffold integration, immune response, and healing.15-27 In parallel, diagnostic barriers such as delayed detection and disparities in access to imaging or genetic testing may influence not only treatment planning but also the timing and success of reconstruction. By including these dimensions, this paper emphasizes that reconstructive decisions are shaped not only by surgical technique or product selection but by a broader biological and diagnostic landscape.28-33
Healthy tissue
Breast tissue is a dynamic, hormonally responsive organ composed of adipose-rich stroma, branched ducts, and terminal ductal lobular units (TDLUs), the latter being the primary site of milk production and hormonal responsiveness.15,16 Structurally, the adult breast is anchored by Cooper’s ligaments to the chest wall and exhibits a heterogeneous mix of adipose and fibroglandular tissue, with the latter predominating in women with dense breasts.16,17 In dense tissue, fibroglandular content can approach 70%, while non-dense breasts are largely composed of adipose tissue, exceeding 80% in some cases.18 Beyond its role in energy storage, adipose tissue in the breast serves as an endocrine and immune organ, hosting stromal cells, fibroblasts, and macrophages that interact with epithelial components to regulate local signaling and homeostasis.19,20
At the extracellular level, healthy breast tissue is scaffolded by a compliant extracellular matrix (ECM) composed of collagen types I, III, and IV, fibronectin, laminin, hyaluronic acid, and matrix metalloproteinases (MMPs), which together provide mechanical and biochemical cues critical for ductal elongation and epithelial polarity. During developmental phases such as puberty or pregnancy, the ECM undergoes dynamic remodeling to accommodate tissue expansion, while maintaining a soft, fibrous architecture distinct from the stiff, fibrotic ECM associated with malignancy.15,20 Mechanical compliance in the healthy breast not only facilitates tissue morphogenesis but also allows for the diffusion of hormones and nutrients necessary for functional differentiation.15,21
The cellular landscape of the healthy breast includes luminal and basal epithelial cells, myoepithelial cells, adipocytes, fibroblasts, and tissue-resident macrophages, all of which contribute to immune surveillance and hormonal crosstalk (Figure 1).15 Metabolic profiling of dense but non-cancerous breast tissue reveals significantly elevated levels of glutamine, taurine, choline, and myo-inositol, which are markers of active metabolism and cell turnover, likely reflective of heightened hormonal stimulation or local inflammation.20 During puberty and early childhood, this activity is mirrored by ductal budding, epithelial proliferation, and early acinar development, triggered by fluctuations in estrogen, FSH, and prolactin.16 Similarly, in adolescent obesity, altered expression of CSF-1 and IL-10 suggests a pre-inflammatory state with implications for future tissue remodeling and cancer susceptibility.18
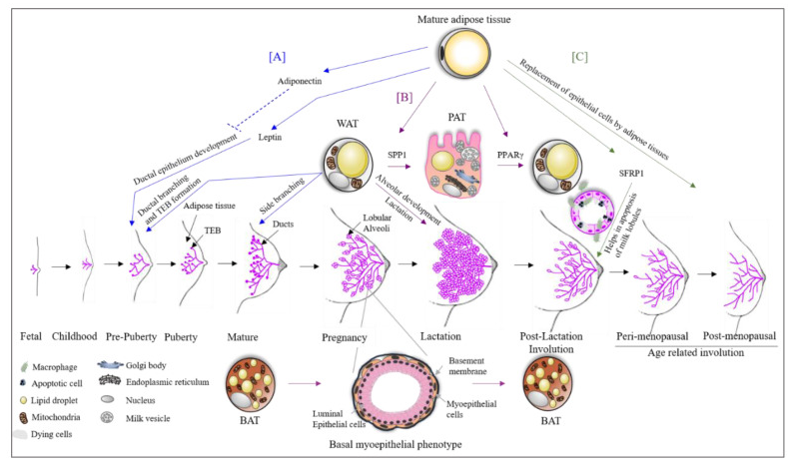
Figure 1 Adipose tissue dynamics during breast development.
A) Leptin secreted by mature adipocytes promotes ductal growth. B) During pregnancy, white adipose tissue (WAT) converts into milk-secreting pink adipose tissue (PAT). C) During involution, epithelial structures are replaced by adipose tissue.
Vascularization plays a central role in supporting these processes, with capillary-rich TDLUs ensuring rapid delivery of oxygen and nutrients during periods of heightened cellular activity.15 In dense breast tissue, the need for vascular support increases further, correlating with the elevated metabolic demands seen in high-density MR spectroscopy signatures.18 Estrogen and VEGF signaling drive angiogenesis, particularly during the larche and lactation, underscoring the need for adequate perfusion to maintain tissue integrity and hormonal responsiveness.16,18
Hormonal influence in the healthy breast begins as early as infancy, with transient estrogen and gonadotropin surges leading to temporary glandular development in both sexes.16 Throughout puberty, rising estrogen levels initiate ductal elongation, while progesterone promotes side branching and lobular formation. Postnatally, prolactin, oxytocin, and IGF-1 regulate milk production and epithelial maintenance.15,16 Aromatase activity in breast adipose tissue, modulated by leptin, IL-6, and PGE2, contributes to localized estrogen production, creating tissue-specific hormonal microenvironments.17,19 Differences in metabolite concentrations between pre- and postmenopausal women, particularly in dense breasts, further illustrate how hormonal shifts modulate tissue activity over the lifespan.20
These structural, cellular, and hormonal features have direct implications for tissue-engineered scaffold and implant design. Biocompatible materials must replicate the compliant, bioactive nature of native ECM while supporting ductal morphogenesis and vascular ingrowth. Composite hydrogels integrating fibronectin, collagen, and laminin have shown promise in recapitulating healthy branching architecture and supporting hormone-responsive cells in 3D culture.48 Moreover, scaffold stiffness and degradation rate must be carefully tuned to accommodate tissue remodeling during different developmental or hormonal phases.15,21 Pediatric or adolescent reconstructions may benefit from softer, more dynamic matrices that mimic the pliability of pubertal breast tissue. Finally, bioengineered solutions must avoid triggering chronic inflammation, particularly in metabolically active or hormonally sensitive environments, where excess estrogen or cytokine signaling could promote fibrosis or hyperplasia.16,20
Diseased tissue
In contrast to the compliant, metabolically stable environment of healthy breast tissue, diseased breast tissue, particularly in the context of malignancy, undergoes significant structural, cellular, and immunological transformations that complicate tissue engineering strategies (Figure 2).22 Cancerous breast tissue is characterized by increased stiffness, abnormal ECM remodeling, and disrupted tissue polarity, all of which interfere with normal ductal organization and morphogenesis.22,23 Key transitions include enhanced deposition of fibrillar collagens, desmoplastic stroma formation, and increased expression of MMPs, which degrade native ECM and facilitate invasion. These modifications result in a biomechanically hostile microenvironment that limits nutrient diffusion, distorts cell signaling, and undermines scaffold integration.23
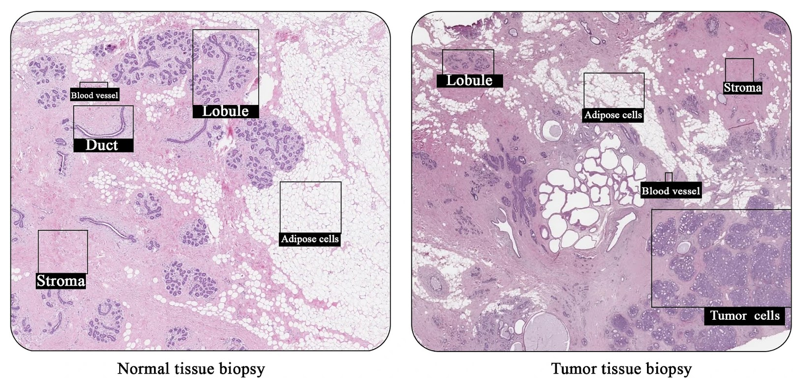
Figure 2 Histological comparison of healthy and cancerous breast tissue.
Microscopic images compare normal ductal breast architecture with a sample from a 68-year-old female with ductal carcinoma grade 1, highlighting differences in tissue organization, density, and pathology.
One of the most pronounced features of diseased breast tissue is the emergence of a pro-tumorigenic microenvironment driven by reciprocal interactions between malignant epithelial cells and surrounding stromal components.55 Tumor-associated fibroblasts (TAFs), for example, secrete ECM components and growth factors like TGF-β, IL-6, and VEGF, which stiffen the tissue and induce epithelial-to-mesenchymal transition (EMT), enhancing invasiveness and metastatic potential. These fibroblasts also play metabolic roles: they engage in aerobic glycolysis and secrete lactate and alanine that feed cancer cell proliferation, while simultaneously establishing nutrient and oxygen gradients that impair immune cell infiltration and engineered scaffold integration.22,23
Compounding the structural challenges is the immune dysfunction that defines the tumor microenvironment. Although breast tumors are often infiltrated by immune cells, their activity is skewed toward immunosuppression due to the dominance of regulatory T cells (Tregs), tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs), which impair cytotoxic responses. These immunosuppressive actors secrete cytokines such as IL-10, VEGF-A, and IP-10, which suppress T cell activation and simultaneously promote angiogenesis, allowing tumors to evade immune surveillance. Tumor cells also exploit checkpoint pathways like PD-L1 and CTLA-4 to disarm cytotoxic T lymphocytes and maintain an immunologically silent niche.24
Recent studies have revealed that diseased breast tissue hosts a distinct microbiome that contributes to immune modulation and may directly impact scaffold-host interactions. Compared to healthy tissue, tumor samples show reduced microbial diversity and are often dominated by pro-inflammatory taxa such as Pseudomonas, Proteus, and Porphyromonas, which have been linked to cancer-promoting inflammation. In contrast, commensals such as Propionibacterium and Staphylococcus, typically present in healthy breast tissue, are markedly depleted in tumors and have been associated with T cell activation and anti-inflammatory signaling. These microbial shifts are accompanied by altered toll-like receptor (TLR) expression patterns in tumor tissue, including downregulation of TLR4 and upregulation of MYD88 and IRAK1, which may impair innate immune recognition of bioengineered implants.25
At the genetic level, breast tumors exhibit widespread chromosomal aberrations and somatic mutations that differ by subtype and histological grade. Amplifications of 1q, 8q, and 17q (e.g., ERBB2), deletions in 16q, and gains in 11q are frequently observed in high-grade cancers like medullary and metaplastic carcinoma, contributing to their aggressive behavior and genomic instability. Conversely, special histological types such as mucinous or adenoid cystic carcinoma display relatively simple genomic profiles, reinforcing the need to account for histologic and molecular heterogeneity when designing scaffolds.26 Moreover, even tissue adjacent to tumors can exhibit “field cancerization,” marked by DNA methylation changes and low-level mutations, which broadens the scope of affected areas that tissue engineers must address.27
Fibroblasts in the tumor stroma also undergo epigenetic reprogramming, supporting metabolic reorganization of the tissue as a whole. Tumor cells shift toward glycolysis and glutaminolysis, producing lactate and depleting oxygen to acidify the ECM and inhibit immune activation. Stromal cells like CAFs participate in this process by supplying alternative fuels and growth signals, further driving cancer cell proliferation and resistance to therapy. These metabolic constraints impose harsh conditions on any introduced biomaterial, requiring resistance to oxidative stress, low pH, and fluctuating nutrient availability.22.23
Finally, breast cancer evolves under the pressure of systemic therapies, leading to additional mutations, epigenetic drift, and shifts in the tumor microenvironment that may render scaffolds obsolete or dysfunctional over time. Immune cells and fibroblasts often persist after treatment, continuing to shape the post-treatment environment in ways that inhibit regenerative success.23,24 These persistent changes highlight the need for implantable materials that are not only structurally supportive but also biologically adaptable to evolving pathophysiology.27
Diagnosis and detection challenges
Effective breast cancer detection and diagnosis rely on a blend of clinical examination, imaging, and genetic risk assessment, tailored by patient age, density of breast tissue, and family history.28 For average-risk individuals, mammography remains the gold standard, typically beginning between the ages of 40–50, with biennial intervals suggested by most guidelines. In women with dense breasts or high-risk profiles, MRI and ultrasound provide enhanced sensitivity and should be considered as part of a risk-adapted screening strategy.30 Triple assessment-a combination of clinical exam, radiological imaging, and biopsy-remains the cornerstone of diagnostic workup following suspicious findings.31 For individuals under 40, dense breast tissue may obscure mammographic accuracy, and adjunct imaging or biopsy is often needed to achieve diagnostic clarity. Increasingly, risk calculators and genetic panels are used to guide both screening and prevention, especially in patients with BRCA mutations or a strong family history.28,29
Despite technological progress, diagnostic delay remains a persistent problem, especially in younger women who present with aggressive tumors that may be misinterpreted as benign conditions like fibroadenomas or cysts. These delays often stem from both patient factors, such as underestimation of risk, and provider-related barriers, including failure to escalate investigation despite concerning symptoms.32 Alarmingly, racial and ethnic disparities further widen the diagnostic gap: Black women are more likely to experience delays in biopsy, staging, and treatment initiation, independent of insurance status. These systemic inequities contribute to higher rates of late-stage diagnoses and poorer outcomes in marginalized populations.33 Additionally, awareness and access to advanced screening tools such as MRI remain disproportionately limited in underserved communities, exacerbating diagnostic disparity.30
Importantly, many of these challenges could be mitigated with structured education, better provider training, and broader implementation of personalized screening models that incorporate genetic and lifestyle risk factors.29,31 As tissue engineers look toward implantable diagnostics or therapeutics, understanding these front-end failures in detection is essential to designing technologies that can intervene earlier or adapt to a range of patient profiles.28-33
Global breast reconstruction market
The global breast reconstruction surgery market is experiencing rapid expansion.34 In 2024, it was valued at 4.5 billion USD, and it is projected to reach USD 10.12 billion by 2035, growing at an estimated compound annual growth rate (CAGR) between 6.5% and 7.5% over the forecast period (Figure 3A).15 This market includes products and services such as breast implants, acellular dermal matrices (ADMs), synthetic meshes, tissue expanders, and regenerative tissue engineering technologies.16 Regional segmentation reveals that the United States currently leads in market share (Figure 3B), driven by high procedural volume, favorable insurance coverage, and wide clinical access to advanced techniques.14 In Europe, particularly Germany and the U.K., demand is growing due to widespread use of synthetic meshes like TiLOOP® Bra and patient-centered innovations such as minimally invasive flap procedures.35 The Asia-Pacific (APAC) region is expected to experience the fastest growth due to rising breast cancer incidence (Figure 3B), increasing healthcare spending, and expanded access to modern reconstructive techniques in countries like China, Japan, and South Korea.36
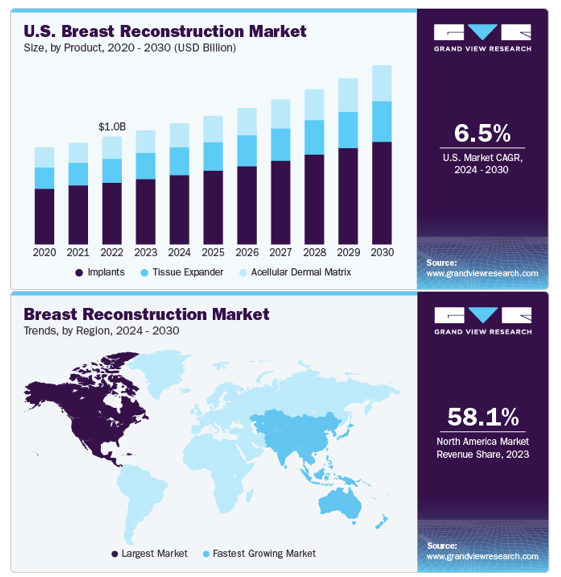
Figure 3 Breast reconstruction market statistics.
A) Line graph showing projected U.S. breast reconstruction growth by product from 2020 to 2030. B) Global map of regional trends, indicating highest growth in the U.S. and Asia-Pacific regions.
The most fundamental driver of breast reconstruction demand is the rising global burden of breast cancer, which remains the most commonly diagnosed cancer among women.34 Over 2.3 million new cases are identified annually, and as screening improves, the number of women eligible for mastectomy and therefore reconstruction is increasing.4 In many cases, women with high-risk genetic mutations (e.g., BRCA1/2) elect for prophylactic mastectomy even in the absence of cancer, expanding the population seeking preventive reconstruction.6 As life expectancy increases and treatment outcomes improve, the population of long-term survivors seeking reconstruction also grows, shifting the conversation from life-saving treatment to quality-of-life restoration.12
Legislative efforts and patient advocacy have significantly increased awareness and acceptance of reconstruction.7 For example, the Women’s Health and Cancer Rights Act (WHCRA) of 1998 in the U.S. mandated that insurance companies cover reconstruction after mastectomy, resulting in a marked increase in procedure rates.7 Public health campaigns, such as National Breast Reconstruction Awareness (BRA) Day and private initiatives like The Reblossom Project by Allergan, have also empowered patients to consider reconstruction as part of their recovery journey.37 Between 2005 and 2014, immediate breast reconstruction (IBR) rates in the U.S. increased significantly, from approximately 26% in 2005 (as seen in Figure 4) to 40–60% by 2014, depending on population subgroup. By 2014, rates reached as high as 63% among patients with ductal carcinoma in situ (DCIS) undergoing mastectomy. Although disparities remain, this growth indicates increasing visibility and normalization of reconstructive care.14
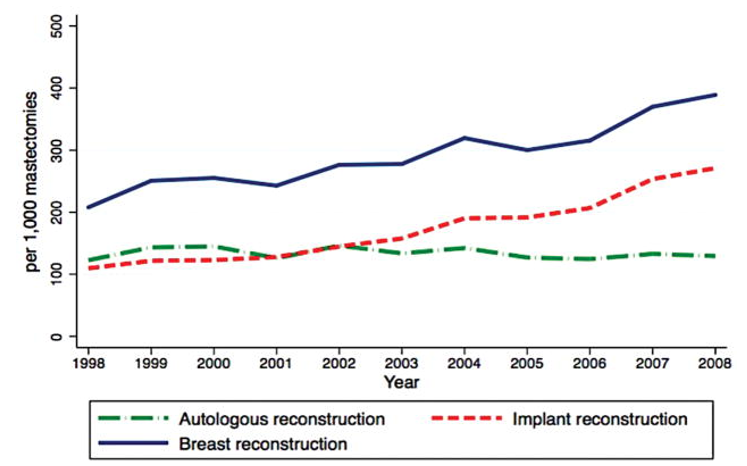
Figure 4 Temporal trends of breast reconstruction following mastectomy.
Graph depicting reconstruction rates and modality use between 1998 and 2008, reflecting policy and cultural shifts in reconstructive decision-making.
The introduction of prepectoral implant placement, direct-to-implant (DTI) procedures, and nipple-sparing mastectomies has significantly improved the aesthetic and functional outcomes of reconstruction, leading to shorter recovery times and greater patient satisfaction. Acellular dermal matrices (ADMs), both human- and animal-derived, have become standard tools in implant-based reconstruction (Figure 5), supporting better tissue integration and reducing the risk of capsular contracture.38 However, due to high costs, synthetic meshes such as TiLOOP® Bra and TIGR® are gaining popularity as more affordable and biocompatible alternatives.35,39 More recently, technologies such as 3D bioprinting, bioresorbable scaffolds, and adipose-derived stem cell (ADSC) therapies are being introduced into clinical trials, representing a major shift toward regenerative, personalized reconstruction.1,3
Market challenges
High product cost remains a barrier to equitable access. While ADM products can cost over $2,000–3,000 per case, synthetic mesh alternatives range between $300–500, and are gaining favor in countries with constrained health budgets or cost-conscious health systems.40 Disparities in access are persistent and well-documented: patients in rural or low-income regions, as well as racial and ethnic minorities, are significantly less likely to receive reconstruction. This reflects differences in referral patterns, surgeon availability, insurance status, and cultural factors.14 Cultural and personal aversions to reconstruction also play a role. Some patients opt out due to concerns over implant safety, religious objections to animal-derived ADMs, or a desire to “go flat” for identity, comfort, or trauma-related reasons.41,42 Even in high-income countries like the U.K., only 30% of mastectomy patients undergo reconstruction, showing that informed choice, not just availability, plays a key role in uptake.
Existing products on the market
Implant-based products
Breast implants remain the cornerstone of implant-based reconstruction, with two primary types in use: saline and silicone (Figure 6A).4 Saline implants consist of a silicone shell filled with sterile saltwater, offering adjustability and requiring a smaller incision since they are filled post-insertion. However, they are more prone to visible wrinkling and are often perceived as less natural in feel.
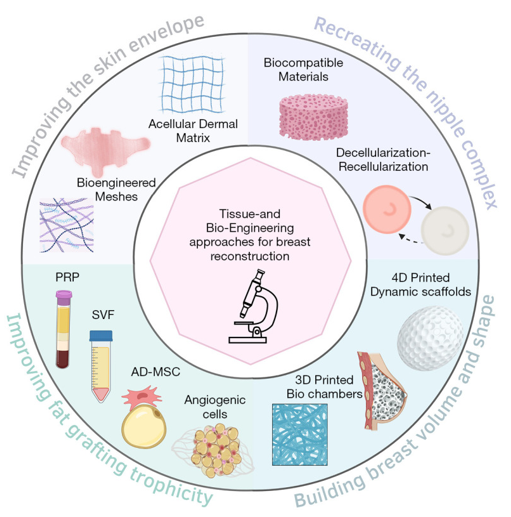
Figure 5 Bioengineering approaches in breast reconstruction.
Illustration summarizing surgical, scaffold-based, and regenerative medicine techniques, including implants, ADMs, and 3D-printed constructs.
Silicone implants are prefilled with cohesive silicone gel that more closely mimics the consistency of natural breast tissue.4 Leading FDA-approved brands include Mentor MemoryGel®, Natrelle® by Allergan, and Sientra OPUS® (Figure 6B-D).43,44 These implants have high satisfaction rates but require periodic MRI surveillance to detect silent ruptures, which can occur without visible symptoms.44,45

Figure 6 Types of breast implants.
A) Comparison of implant shapes and profiles. B–D) Examples of Mentor MemoryGel®, Natrelle® by Allergan, and Sientra OPUS® silicone implants. Visuals highlight shell structure and fill composition.
Structured saline implants, such as the Ideal Implant®, were developed to provide an alternative to traditional unstructured saline and silicone gel implants (Figure 7A). According to the 10-year core study, the Ideal Implant combines the safety and detectability of saline with a feel that more closely resembles silicone gel. Its design features an internal structure of nested baffle shells that reduce fluid movement, which creates a firmer, more natural-feeling implant and minimizes wrinkling (Figure 7B). Because the filler is saline, rupture is visually detectable without the need for routine MRI screening. The implant’s layered shell structure also enhances strength and durability. Although the Ideal Implant was introduced to address drawbacks of existing options, its adoption may require more patient and surgeon education due to its unique category within the market.46
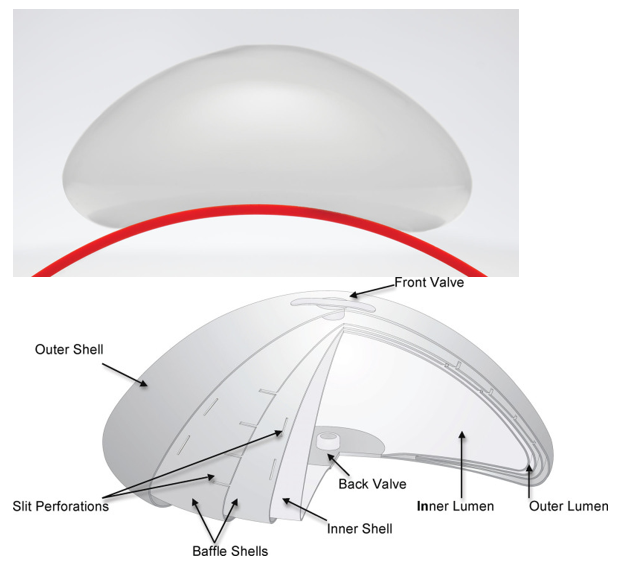
Figure 7 IDEAL structured saline implant.
A) Photograph of IDEAL Implant. B) Cross-sectional schematic showing nested baffle shells designed to reduce fluid sloshing and improve feel.
Silicone implants are praised for their natural look and tactile realism, making them the most commonly used option; however, they come with risks of silent rupture and require ongoing imaging surveillance.13,44 In contrast, saline implants are less expensive and easier to monitor for rupture, though they may produce inferior aesthetic results due to their fluid content. Structured implants offer a compromise between the two but have not been as widely adopted due to higher cost and more limited clinical familiarity.46
As seen in Figure 8A, ADMs are biologically derived scaffolds made from decellularized human (AlloDerm®), porcine (Strattice™), or bovine (SurgiMend®) dermis.40,47 These sheets are used to reinforce the implant pocket, support the lower pole of the breast, and improve outcomes in prepectoral and subpectoral implant placement. ADMs are especially useful in direct-to-implant (DTI) procedures and are associated with better soft tissue integration, improved contouring, and reduced incidence of capsular contracture.1,47
Some studies, like the CARE trial and BROWSE study, have shown that ADMs reduce long-term contracture, malposition, and postoperative pain.1 However, others report increased risks of infection and seroma, particularly in patients with obesity, smoking history, or diabetes.47
Tissue expanders are temporary devices inserted post-mastectomy to gradually stretch skin and muscle, creating space for a future permanent implant. Mentor Spectrum™ expanders are commonly used (Figure 9A), while AirXpander’s AeroForm® represents an innovative approach using CO2 gas and a handheld controller to allow patients to self-administer expansions at home (Figure 9B).48 This technology significantly reduces the need for frequent clinical visits and may improve comfort and autonomy.38,48

Figure 8 Acellular dermal matrix (ADM) products used in reconstruction.
A) Diagram showing human, porcine, and bovine sources of ADMs. B) Image of a hydrated AlloDerm® sheet, commonly used for soft tissue support in implant procedures.
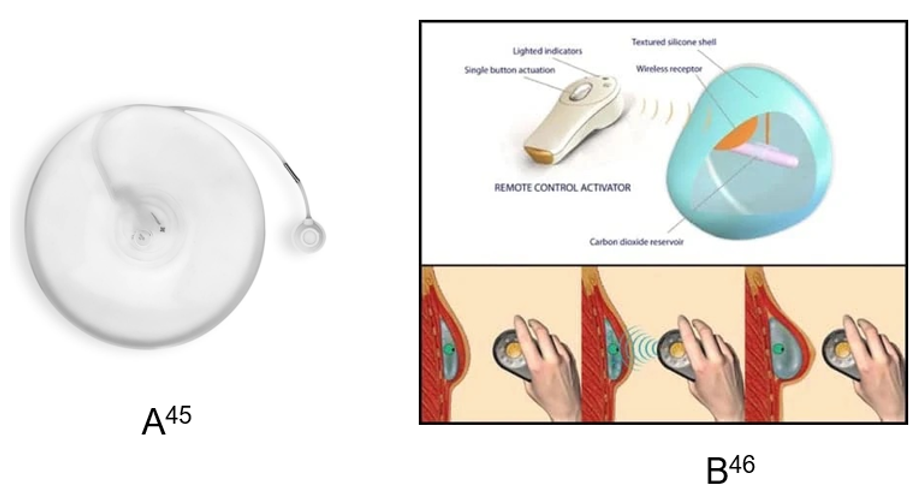
Figure 9 Tissue expansion devices.
A) Mentor Spectrum™ expander used in staged reconstruction. B) AeroForm® expander with a handheld device allowing patients to control CO₂ inflation at home.
Summary
Acellular dermal matrices offer important mechanical and aesthetic advantages by providing improved soft tissue support and enhancing the integration of implants, especially in challenging reconstructions. However, their high cost and potential for complications like infection or seroma limit their universal use, especially in resource-constrained settings.4,38 Tissue expanders remain essential in two-stage reconstructions, and innovations like AeroForm® CO2 expanders enhance patient experience by reducing clinical burden and expansion-related discomfort.38,49
A transformative advancement in breast reconstruction involves 3D-printed, bioresorbable scaffolds designed to regenerate adipose tissue while providing mechanical support. Products like Mat(T)isse™ by Lattice Medical (Figure 10) and Senella™ by BellaSeno GmbH are made from polycaprolactone (PCL) and are tailored to the patient’s anatomy.1 These scaffolds are implanted in place of a permanent implant and serve as a temporary matrix for cell infiltration, vascularization, and fat ingrowth. In early studies, PCL scaffolds combined with autologous fat or ADSCs demonstrated over 80% volume retention and angiogenic integration over 6–12 months.2 These approaches are promising alternatives for patients who prefer not to use traditional implants.1,50
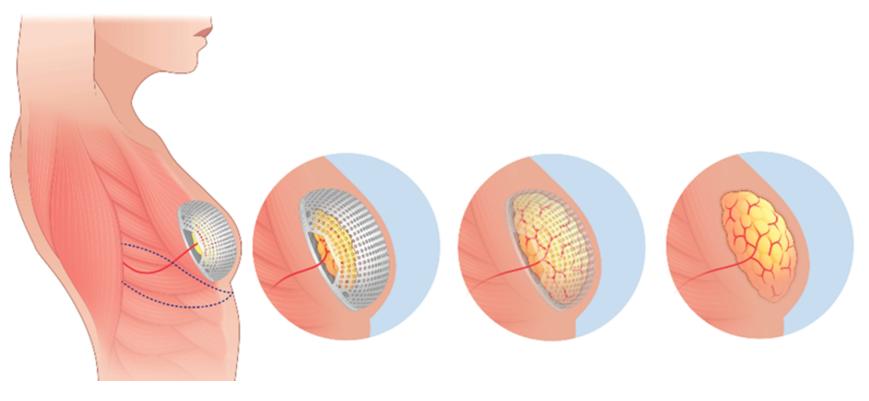
Figure 10 Mat(T)isse™ 3D-printed scaffold by lattice medical.
Image of a PCL-based scaffold seeded with adipose tissue for regenerative breast reconstruction. The structure is designed to degrade as native tissue grows.
These synthetic polymers are commonly used in experimental scaffold design due to their biocompatibility, tunable degradation rates, and mechanical strength. For example, PCL is known for its slow resorption rate and ability to support adipose regeneration, while PLA offers structural integrity and customizable porosity. Composite scaffolds can also be seeded with growth factors (VEGF, bFGF) or ADSCs to enhance vascularization and fat formation. Although primarily used in research or limited clinical trials, these constructs represent a next-generation, implant-free approach to breast reconstruction, especially for patients seeking natural results or those with contraindications to silicone-based products.1,50
3D-printed scaffolds like Mat(T)isse™ and Senella™ represent a cutting-edge shift toward personalized, regenerative breast reconstruction.1 These implants are biodegradable, eliminate the need for silicone, and can be custom-designed to match the patient’s anatomy.3 However, they remain in early-phase trials, are not yet widely available, and may be costly to produce and implement clinically. Composite biomaterials offer versatility and potential for integration with biologics, but more data are needed on long-term outcomes and regulatory approval pathways before they become mainstream options.50
Cell-assisted lipotransfer (CAL)
Developer / Institution: University of Tokyo (Dr. Kotaro Yoshimura); RESTORE-2 trial in Europe.
Trial phase: Phase II observational (RESTORE-2)
Completed summary of trial: CAL is a method of fat grafting enhanced by adding stromal vascular fraction (SVF) or ADSCs to improve graft retention, angiogenesis, and integration.33 The RESTORE-2 clinical trial showed approximately 80% volume retention at 12 months, 85% patient satisfaction, and no oncologic recurrence. Supporting preclinical studies, including those by Kolle et al., showed similarly strong fat retention and vascular growth in both humans and animals.51
How it differs: Traditional fat grafting often results in unpredictable volume loss due to poor vascularization. CAL addresses this by enhancing the survival and regenerative potential of grafts, essential for patients with radiated or scar-compromised tissue.51
Developer / institution: lattice medical (France)
Trial phase: First-in-human clinical trial launched in July 2022 in Georgia (Europe)
Summary of Trial: Mat(T)isse™ is a 3D-printed scaffold made of polycaprolactone (PCL) that supports autologous fat integration after single-stage implantation.1,32 It resorbs over 12–18 months, and animal models (e.g., rabbits) have shown over 90% adipose volume retention after one year.2, 23 The device is currently undergoing clinical testing in humans to monitor volume, biocompatibility, and foreign-body response.1,52
How it differs: Unlike traditional silicone implants, Mat(T)isse™ offers a fully degradable scaffold that regenerates autologous tissue, thereby avoiding permanent foreign materials and reducing complications like capsular contracture.1,53
Developer / Institution: FixNip NRI™ (Israel); other teams in Europe and the U.S.
Trial phase: preclinical (animal studies)
Summary of trial: Bioengineered NAC solutions include decellularized porcine tissue scaffolds, 3D-bioprinted hydrogels, and silicone-based implants (e.g., FixNip NRI™). These approaches aim to restore long-term projection, pigmentation, and sensation.1,54 In animal studies, several NAC implants demonstrated successful vascularization and early neural ingrowth, suggesting viability for human use in the near future.1
How it differs: Current clinical options, like tattooing or skin flaps, often result in projection loss and a lack of sensation. Bioengineered NAC implants address these limitations by offering structural stability, regenerative potential, and neurovascular integration.1
Developer / Institution: healshape (France)
Trial phase: Preclinical; first-in-human trial planned by 2025
Summary of trial: Healshape’s injectable hydrogel forms a scaffold after fat injection, which gradually degrades over 6–9 months as new adipose tissue fills the space. Preclinical rodent models have shown strong biocompatibility, scaffold resorption, and adipogenesis.50,53 This injectable system is designed for minimally invasive reconstruction, particularly in small to moderate volume defects.
How it differs: This hydrogel scaffold replaces more invasive surgeries and permanent implants with a resorbable, scaffold-guided regeneration approach, providing structural support through natural healing processes.53,55
Interi™ manifold drain system
Developer / Institution: IC Surgical (device); Stanford University (sponsor)
Trial phase: Early-phase, randomized pilot trial (estimated completion: September 2025)
Summary of trial: The Interi™ Manifold is a novel, multi-branched internal drain designed for use in implant-based breast reconstruction (IBBR). It aims to address limitations of traditional Jackson-Pratt (JP) drains by providing consistent suction, enhanced fluid evacuation, and improved patient comfort. In the randomized trial, patients undergoing bilateral mastectomy and immediate prepectoral implant reconstruction receive both Interi and JP drains-one per breast-allowing direct comparison within each individual.
How it differs: Traditional JP drains are limited by low capacity, inconsistent suction, and prolonged retention time, which can delay recovery. The Interi™ system offers a closed, multi-lumen design that enables more reliable drainage, potentially reducing the number of drain sites, minimizing complications, and accelerating the timeline for expansion and reconstruction.56
Breast reconstruction is an evolving discipline at the intersection of surgical innovation and tissue engineering, where new technologies are being integrated to improve outcomes across volume restoration, skin quality, and sensation.1 Diverse reconstructive modalities, including implant-based, autologous, and hybrid approaches, allow surgeons to match the technique to the patient’s anatomy, treatment history, and psychosocial goals.7 Implants remain the most widely used method in the United States, but autologous and hybrid reconstructions are increasingly being offered at high-volume or academic centers due to their long-term satisfaction outcomes.10 Patient-reported outcome measures (PROMs), such as the BREAST-Q, consistently show that autologous reconstructions outperform implants in terms of body image, psychosocial well-being, and physical comfort.11 As a result, there is a growing shift toward incorporating more personalized and regenerative approaches into routine care.39
Understanding the biology of the breast is increasingly important in this context. Healthy breast tissue is pliable, vascular, and hormonally responsive, while diseased tissue presents significant challenges like fibrosis, immune suppression, and altered metabolism that can compromise scaffold integration. These pathological differences highlight the importance of customizing reconstructive materials and strategies to fit the tissue environment.15-27 Likewise, early diagnosis and timely surgical planning are crucial, but are frequently compromised by diagnostic delays, imaging limitations, and disparities in care access across race, age, and socioeconomic status. These barriers not only delay treatment but may influence the viability of immediate versus delayed reconstruction.28-33
A key future direction is the development of personalized, bioprinted implants and scaffolds that can be customized to each patient's anatomy and biological environment.3 Biodegradable scaffolds printed from polycaprolactone or hydrogel materials are being designed to support adipose tissue regeneration, gradually resorbing as natural tissue replaces the scaffold.1 These innovations often incorporate stem cells, especially ADSCs, which have shown promise in enhancing graft survival, vascularization, and tissue integration.51-60 In parallel, hybrid strategies that combine implantable scaffolds with biologics like platelet-rich plasma (PRP) or SVF are being explored to offer both mechanical support and regenerative benefits. Despite these technological advancements, access remains inequitable. Patients from underserved racial, economic, and geographic backgrounds are significantly less likely to receive breast reconstruction.14 Expanding access to reconstructive options will require policy reform, surgeon training, and the wider distribution of affordable, scalable technologies.5
Ultimately, the future of breast reconstruction depends on combining technological innovation with patient-centered care: an approach that respects individual anatomy, priorities, and identity.1 The most successful advances will not just reconstruct form, but restore dignity, sensation, and quality of life for people recovering from breast cancer.61-68
There is no funding to report for this study.
Makena Rudy would like to express gratitude to Professor Bill Tawil for mentorship and guidance throughout the development of this manuscript. His insights into tissue engineering, scaffold design, and clinical translation greatly contributed to the depth and clarity of this review. Additional appreciation is extended to the Department of Bioengineering at UCLA for providing a supportive academic environment that fostered the completion of this work.
The authors declare there is no conflicts of interest.

©2025 Makena, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.