Journal of
eISSN: 2475-5540


Review Article Volume 10 Issue 1
Department of Bioengineering, University of California, Los Angeles (UCLA), USA Correspondence: Dr. B
Correspondence: Dr. Bill Tawil, Department of Bioengineering, UCLA School of Engineering, 420 Westwood Plaza, Room 5121, and Engineering V. P.O. Box: 951600, Los Angeles, CA 90095-1600, USA
Received: June 21, 2025 | Published: July 25, 2025
Citation: Helen R, Bill T. A comprehensive review of osteoarthritis: physiology, disease, market analysis, existing products, and prospective products. J Stem Cell Res Ther. 2025;10(1):175-182. DOI: 10.15406/jsrt.2025.10.00203
Osteoarthritis (OA) is a degenerative joint disease and the most common form of arthritis. The disease is classified by the degradation of protective cartilage resulting in painful and limiting symptoms. Osteoarthritis primarily affects members of the elderly population who have a previous history of injuries or diseases including obesity. Cases of OA are most prevalent in the hands, hip, back and knees. There is no cure for OA, however existing treatments can help relieve symptoms such as pain and inflammation. Due to aging populations, market demand for osteoarthritis therapeutics continues to increase, in turn, the demand for novel solutions emerges. This review analyzes the osteoarthritis market size and trends, as well as current and emerging therapeutic products.
Keywords: osteoarthritis, tissue engineering, joint disease, joint replacement, viscosupplementation
OA, osteoarthritis; CAGR, compound annual growth rate; NSAID’s, nonsteroidal anti-inflammatory drugs; NRS, numeric rating scale; WOMAC, western Ontario and McMaster universities; DASH, disabilities of the arm, shoulder, and hand; SF-12, 12-item short form health survey; PFOA, patello-femoral compartment of the knee; ECM, extracellular matrix; N-TEC, nasal chondrocyte tissue engineered cartilage; PRP, platelet rich plasma; KOOS, knee osteoarthritis outcome score; MARS, Marx activity rating scal
Osteoarthritis (OA) is a degenerative joint disease and a form of arthritis where the protective cartilage on the ends of bones wear down over time. The loss of cartilage leads to pain, stiffness, decreased mobility, bone spurs, and swelling. Osteoarthritis most commonly affects the hands, hips, back, and knees and is associated with risk factors like old age, obesity, injuries, or pre-existing disease.1,2 Since osteoarthritis is a chronic disease, there is currently no cure, but there are treatments involving self-management, medications, or, in more extreme cases, surgery to relieve symptoms.3 Currently, OA affects 528 million people worldwide in 2019 and is expected to grow due to aging populations. Thus, market demand for osteoarthritis therapeutics is projected to increase and the need for novel solutions emerges.4 This review aims to discuss the market size and common trends in OA, as well as analyzing current and emerging therapeutic products. Current products include non-invasive preventive and protective measures, nonsteroidal anti-inflammatory drugs, various injections, or surgery.3 Emerging products incorporate novel drugs, techniques, and tissue engineering approaches to hopefully introduce more effective and long-term solutions.5,6
Joints are crucial to the mechanical function of the human body. They are responsible for stabilizing and enabling movement. A joint is the point where two bones make contact. Joints are made of bones and connective tissue and develop from mesenchyme. Muscles help support and stabilize joints. Joints can be classified by their function and type of connective tissue. There are 3 functional classifications: synarthrosis (immovable), amphiarthrosis (slightly movable), and diarthrosis (freely movable). Joints are also classified structurally into fibrous, cartilaginous, and synovial joints.7
Fibrous joints
Fibrous joints are fixed, immovable joints (synarthrosis). The bones are joined together by dense collagenous fibrous connective tissue and lack a joint cavity. There are three types of fibrous joints including sutures, syndesmoses, and gomphoses. Some examples of each are skull bones, distal tibia-fibula joint, and tooth in socket, respectively.7
Cartilaginous joints
The bones in cartilaginous joints are attached by hyaline or fibrous cartilage and lack a joint cavity. Cartilaginous joints can be differentiated into primary and secondary. Primary cartilaginous joints or synchondrosis are joints only connected by hyaline cartilage, for example epiphyseal plates. Secondary cartilaginous joints or symphysis are joints only connected by fibrocartilage, an example is the intervertebral discs. Symphysis joints are typically amphiarthrosis.7
Synovial joints
Synovial joints are the most mobile joints, making them the most functional type of joint in the body. Synovial joints are defined by the presence of a fluid-filled joint cavity. This allows for minimized friction between articulating bones when moving (39). The joint cavity is surrounded by an articular capsule. The capsule is made of fibrous connective tissue that attaches to the bones in the joint just beyond the articulation surface. The fluid within the joint cavity is secreted by the synovial membrane, which lines the articular capsule. The articular cartilage is formed by hyaline cartilage and covers the articulating surface of the bone, it is continuous with the synovial membrane. Some synovial joints contain fibrocartilage between articulating bones, such as the knee menisci. Synovial joints are diarthroses but can be further classified by the types of movements they allow. As shown in Figure 1, the classifications include hinge, saddle, planar, pivot, condyloid, and ball-and-socket.7,8
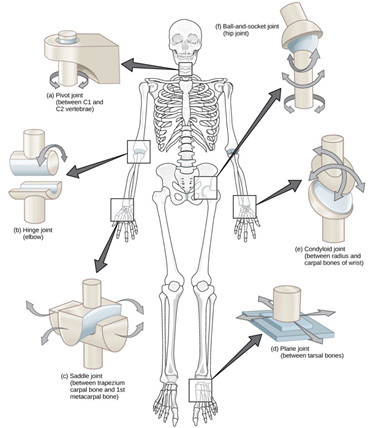
Figure 1 Diagram of the different types of synovial joints: pivot (a), hinge (b), saddle (c), planar (d), condyloid (e), and ball-and-socket (e).8
The image shows the 6 types of synovial joints (pivot, hinge, saddle, planar, condyloid, ball-and-socket) and where the joints might be found.
Hinge joints
Hinge joints are uniaxial and defined by the articulation between a convex and a concave end of each bone. These joints permit flexion and extension and are identified in the elbow, knee, ankle, and interphalangeal (finger) joints.7
Condyloid joints
Condyloid joints are biaxial and involve the articulation between the depressions of one end of the bone and with the rounded ends of one or more bones. Condyloid joints permit flexion, extension, adduction, and abduction. One example are the knuckles, it’s formed by distal metacarpals and proximal phalanges.7
Saddle joints
Saddle joints are biaxial and articulate between two saddle-shaped bones (concave and convex). An example is the thumb joint (trapezium and first metacarpal bone), the joint permits the thumb to flex, extend, abduct, and adduct.7
Planar joints
Planar joints, also known as gliding joints, are multiaxial and consist of two flat bones of similar size (39). Though multiaxial, planar joints are restricted by surrounding ligaments. Some examples of planar joints include acromioclavicular, intercarpal, and intertarsal joints.7
Pivot joints
Pivot joints are uniaxial and the articulation occurs between one bone end and the cylindrical end of another and is enclosed in a ligamentous ring. One example is the proximal radioulnar joint, the articulation allows for pronation and supination.
Ball-and-socket joint
Lastly, the ball-and-socket joint articulates between the rounded head of one bone (ball) and the concavity of the other (socket). This joint is multiaxial, allowing for flexion, extension, abduction, adduction, and rotation. Two examples are the hip and shoulder joints, the ball-and-socket joints permit a wider range of motion compared to other types of joints.7
Embryology
Joints are composed of bones and connective tissues, thus originating from embryonic mesenchyme. The mesenchymal stem cells form osteoblasts which are responsible for creating the bone matrix (intramembranous ossification). Mesenchymal stem cells can also differentiate into chondrocytes which first produce cartilage that gets replaced by bone (endochondral ossification). Osteoblasts, osteocytes, and osteoclasts are responsible for the formation and breakdown of bone.9 The cartilage is maintained by the chondrocytes and the synovial fluid is produced by the mesenchymal cells.7
Nerves
Synovial joints contain nerves that serve sensory and proprioceptive roles. Sensory nerves (proprioceptors) lie within the articular capsule and ligaments and provide proprioceptive feedback via Ruffini endings and Pacinian corpuscles. This feedback is responsible for controlling posture, locomotion, and movement. Free nerve endings or nociceptors are responsible for localized pain detection. Articular cartilage does not contain nerves.
The innervation principle, or Hilton’s law, states the articular nerves supplying a joint also are the nerves supplying the muscles that move that joint and the skin covering the joint. Thus, this allows for reflex coordination and pain reception.7
Muscles
Muscles play a crucial role in synovial joints as they provide support and stability. Muscles and tendons help resist or counteract the forces applied to the joint. Synovial joints are susceptible to instability or dislocation, thus muscle strength is essential.7
While there are numerous joint diseases such as rheumatoid arthritis, gout, or bursitis, this review focuses on osteoarthritis. Osteoarthritis (OA) is the most common form of arthritis and is a non-inflammatory, degenerative joint disease where the articular cartilage wears down as shown in Figure 2.10 The loss of cartilage results in pain, stiffness, swelling, or functional impairment.1
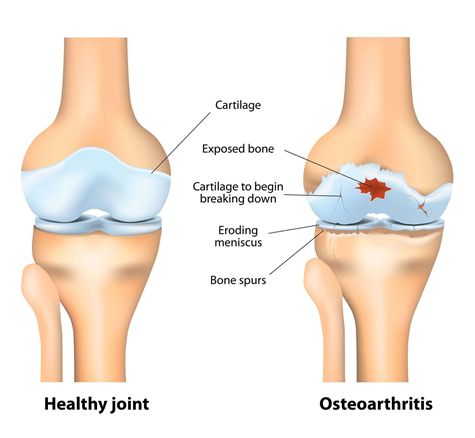
Figure 2 Comparison of a healthy joint (left) with a joint with osteoarthritis (right).10
The image shows an anatomical depiction of a healthy joint compared to a joint affected by osteoarthritis.
The breakdown of the cartilage can be initiated by a variety of factors including mechanical stress, aging, obesity, or injury (1). These factors can cause an imbalance in the cartilage microenvironment such as inflammation, imbalance of anabolic and catabolic processes, or oxidative stress. This imbalance causes chondrocytes to release matrix-degrading enzymes, leading to the loss of components of the ECM, such as proteoglycans and type II collagen (42). Ultimately, leading to cartilage thinning and loss of elasticity.11 The cartilage itself contains no nerves, however, the thinning of the cartilage stimulates pain fibers in nearby areas. As a response, more synovial fluid may be produced causing swelling and inflammation. The excess synovial fluid will stretch the articular capsule causing pain and stiffness. This may also compress the underlying bone, which causes pain.7 Aside from chronic pain, other side effects of OA include sclerosis, cyst formation, or bone spurs.1,2
OA most commonly affects the hands, hips, spine, and knees and is associated with risk factors like old age, obesity, genetics, sex, injuries, repeated use, weak muscles, or pre-existing disease. Obesity or extra weight will affect one’s metabolism and increase mechanical stress on weight-bearing joints, leading to increased risk of OA. Women are also more at risk for developing OA and may be correlated to menopause or hormone changes. Pre-existing diseases like diabetes or hemochromatosis (too much iron) can increase the risk of OA. OA patients may experience pain, aches, joint stiffness, limited range of motion, clicking sounds, joining swelling, or joint instability.1,2,4
OA can be diagnosed via health history, physical exams, X-rays, or laboratory tests. Since osteoarthritis is a chronic disease, there is currently no cure, but there are treatments involving self-management, medications, or, in more extreme cases, surgery to relieve symptoms.3
Market size
Osteoarthritis is the most common form of arthritis, affecting 528 million people worldwide in 2019 and 32.5 million people in the U.S. in 2023.4,12,13 Due to the advancements in healthcare and overall quality of life, life expectancies are increasing leading to aging populations and increase in rates of obesity and injury. As these are all risk factors for osteoarthritis, the market is expected to continue to increase globally.
In 2024, the global osteoarthritis therapeutics market was valued at USD 9.13 billion and is expected to grow at a compound annual growth rate (CAGR) of 6.89% from 2025 to 2030, as shown in Figure 3.4 The projected increase can also be correlated with the advancements being made in drug delivery systems and other treatments like personalized treatments or cell-based therapies. Promising results in these areas drive investments and as a result, increase in market size. Of the USD 9.13 billion, viscosupplementation accounted for 37.06% of it due to its efficacy in managing OA symptoms.4 As shown by Figure 4, the global viscosupplementation market size alone was estimated at USD 4.41 billion in 2022 and projected to grow at a CAGR of 9.58% from 2023 to 2030 and the U.S. market was valued at USD 1.5 billion in 2021 with a CAGR of 8.8%.14
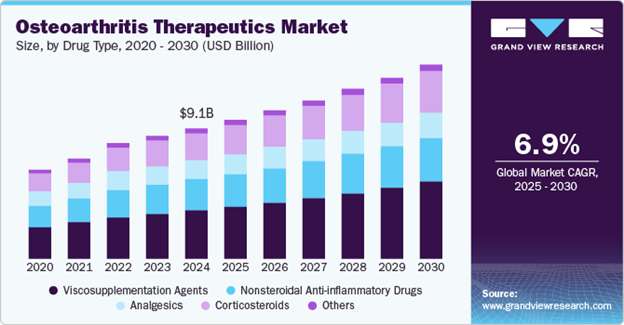
Figure 3 Stacked bar chart displaying the breakdown, CAGR, and USD value of the global osteoarthritis therapeutics market.4
The image shows a stacked bar chart for the global osteoarthritis therapeutics market from 2020 to 2030 (predicted). It is shown that by 2024 the market reached USD $9.1 billion and has a CAGR of 6.9% from 2025 to 2030.
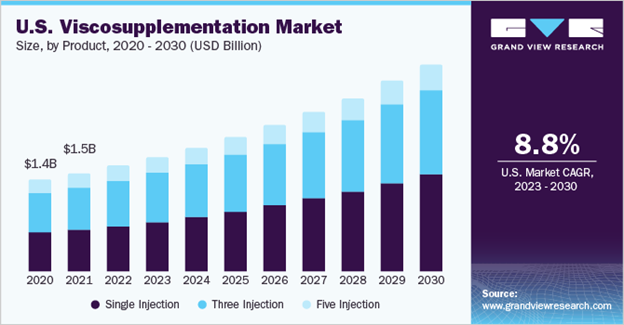
Figure 4 Stacked bar chart displaying the breakdown, CAGR, and USD value of the U.S. viscosupplementation market.14
The image shows a stacked bar chart for the visco-supplementation market in the U.S., which also valued the market at USD $1.5 billion in 2021 and a CAGR of 8.8% from 2023 to 2030.
Figure 5 shows that the OA therapeutics market is dominated by North America, as it is responsible for 37.31% of the market in 2024. This can be linked to the higher prevalence of obesity, supportive government policies, and the presence of leading pharmaceutical/therapeutics companies. In 2023, the annual economic burden in the U.S. was estimated to be USD 136.8 billion.4
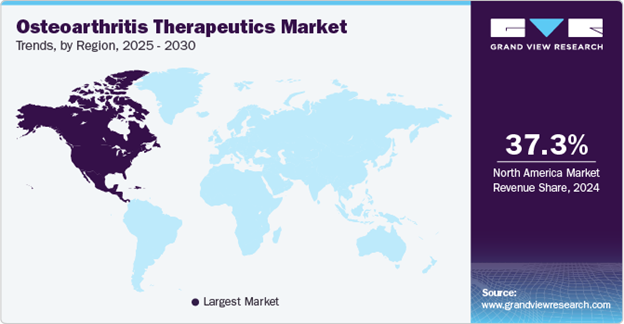
Figure 5 Map highlighting the largest market in osteoarthritis therapeutics.4
The image shows a geographical map of the world highlighting the largest market for osteoarthritis therapeutics, which is North America. It also provides insight that by 2024, the North American market is responsible for 37.3% of the therapeutics market.
Trends
The increase in the global market can also be traced to trends in the Asia Pacific and Europe. Many countries in the Asia Pacific are experiencing an increase in at-risk populations, population growth, lifestyle changes, and increased awareness of OA. On the other hand, European countries are introducing opportunities for companies due to advancements in pharmaceuticals and research for treatments.4
OA tends to affect older populations (>55 years). The most common form of OA is in the knee, the second most prevalent is in the hip, then the hand.12,15 Knee OA made up 42.07% of osteoarthritis cases in 2024.4 The prevalence of knee OA can be correlated to previous injuries, excessive stress from sports or daily activities, genetics, or being overweight.16
Gender is also a contributor to developing OA. It has been observed that women are more susceptible to developing OA compared to men as 73% of people living with OA are older than 55 and 60% of those are female. Specifically, women are 3.5 times, 40%, and 10% more likely to develop hand OA, knee OA, and hip OA (respectively). Studies show that it may be attributed to the hormonal changes women experience during menstruation and menopause.4 During menopause, women will experience a decrease in estrogen which is potentially linked to the degradation of cartilage. Women also have different musculoskeletal systems, higher incidences of injuries (ie. ACL injury), and higher obesity rates. Figure 6 shows that it was also observed that women naturally have less knee cartilage volume compared to men, meaning that women are much more susceptible to knee OA.17,18
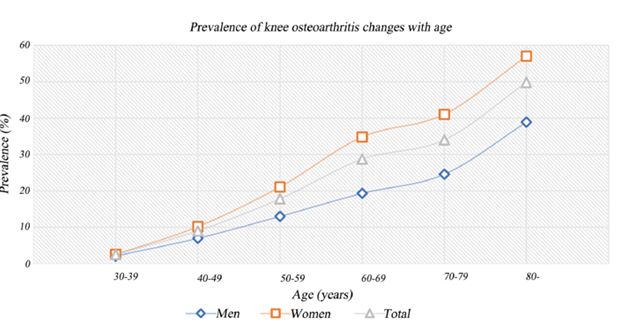
Figure 6 Prevalence of knee osteoarthritis with age and the differences between men and women.18
This image shows a graph that shows the prevalence (%) of knee osteoarthritis in men and women as age increases.
While there are no permanent solutions for osteoarthritis, there are many treatments available to help patients alleviate their pain and symptoms. Currently, the most commonly used forms of treatment include non-invasive preventive/protective methods (i.e. exercise, physical therapy), nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroid or hyaluronic acid injections, and surgery.3
Non-invasive methods
Osteoarthritis patients can also alleviate and manage their symptoms by being physically active, losing weight/maintaining a healthy weight, protecting their joints, physical therapy, and utilizing supportive devices. By maintaining a healthy weight, the patient can decrease the amount of stress carried by the joints and potentially relieve their symptoms. Supportive devices may include crutches, canes, or braces to help redistribute weight.19,20 Knee braces, specifically, can help with knee OA by applying compression or stabilizing the knee. Figure 7 shows a few examples including compression sleeves, hinged brace, and unloader brace.20-23 Non-invasive options tend to be relatively cost efficient and accessible.
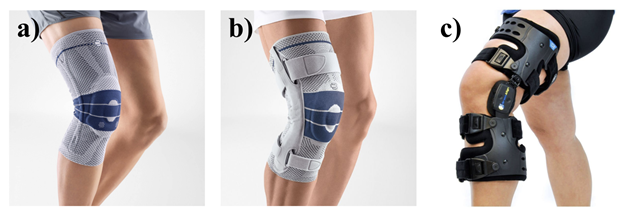
Figure 7 Types of knee braces for knee osteoarthritis a) compression sleeve22 b) hinged brace23 c) unloader brace.21
This image shows three different types of knee braces that are commonly used for knee osteoarthritis. These three braces include the compression sleeve, hinged brace, and unloader brace.
Nonsteroidal anti-inflammatory drugs (NSAIDs)
NSAIDs are commonly used to alleviate the symptoms of OA. These can be found in the form of topical or pill treatments. Topical treatments come in the forms of liquid, patches, or gels. Figure 8 shows commonly used products include Pennsaid (liquid), Flector (patches), and Voltaren (gel).19,24-26 Pennsaid costs around $20-70, Flector costs around $100-400, and Voltaren costs around $15-30.27 Overall, these are inexpensive, accessible options for minor and temporary alleviation.
Pill form treatments include drugs such as ibuprofen, naproxen, and diclofenac. These drugs work to block enzymes that cause pain and swelling, but long-term or frequent use can lead to adverse side effects such as increased risk of heart attack, stroke, and developing ulcers/gastrointestinal bleeding. These treatments are also generally inexpensive and very accessible, with prices starting as low as $2 (cost is dependent on pill count).27 Both topical and pill treatments are either prescribed by a doctor or can be purchased over-the-counter.19
Injections
Another common form of treatment for OA are injections into the affected joint (intra-articular). The two most common injections are corticosteroids and hyaluronic acid (viscosupplementation). Corticosteroids are steroid injections aimed to reduce swelling and relieve pains for a few months.19 These steroid injections are shown by Figure 9.28 These are synthetic drugs that mimic cortisol, which are responsible for regulating immune response and inflammation levels. Thus, the corticosteroids work by suppressing the immune system and reducing inflammation to provide pain and swelling relief. However, since it is a steroid treatment, patients are limited to 3-4 shots a year and may experience weight gain, mood swings, mild weakness, and many other side effects.19,30 Additionally, subsequent shots may not be as effective as the first shot. The cost of an injection can cost anywhere from $40 to $650, depending on location, insurance, and the healthcare provider.29
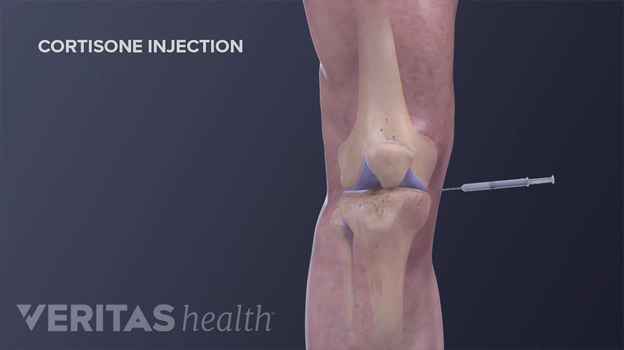
Figure 9 Intra-articular corticosteroid injection administered to the knee.28
This image shows a graphic of a cortisone injection being administered to the knee.
Hyaluronic acid injections or viscosupplementation are used to increase lubrication and cushioning to the affected joint. Figure 10 shows an example of viscosupplementations.31 Hyaluronic acid is essential for healing wounds, regenerating scar tissue, and can be found in cartilage and synovial fluid. It aims to temporarily increase the concentration of joint fluid and act as an anti-inflammatory. The hyaluronic acid can be made artificially or xenogenic via the combs on rooster/hen heads. Patients can either receive a single shot, 3, or 5 injections. Higher doses of hyaluronic acid may cause more side effects like swelling or pain, but have more potential in lubricating the joint. These injections are administered by a clinician and the patient can go home after the procedure. Patients may experience temporary inflammation and less commonly bleeding, blistering, numbness, ulceration, or injection flares. Depending on the patient's insurance, it costs, at least, $300 per injection out-of-pocket. Overall, there is limited proof of efficacy and results may be inconsistent.19,32
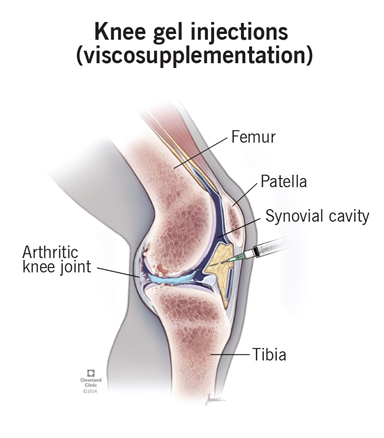
Figure 10 Intra-articular viscosupplementation injection administered to the knee.31
This image shows an anatomical view of a viscosupplementation injection being administered to the knee intra-articularly.
Surgery
Surgery is often used as a last resort for osteoarthritis patients.2 The main procedures performed are osteotomies and partial or total joint replacement.1 Osteotomy is the surgical removal of a piece of bone.33 In the case of knee osteotomy, as shown by Figure 11, the surgeon might remove or add a piece of bone into the shin or thigh to shift weight to an undamaged portion of the joint, essentially correcting the joint alignment. There are several risks associated with osteotomy such as infections, blood clots, improper healing, joint inflammation, stiffness, and long term pain. It can also take a couple months for the surgical site to fully heal and will likely require supportive devices and physical therapy.34
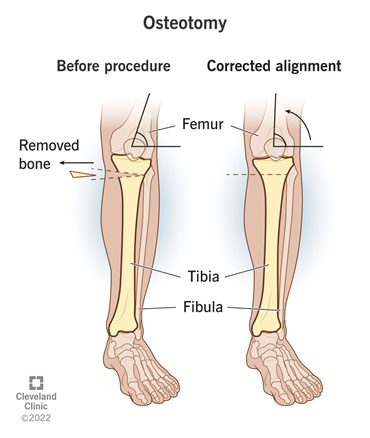
Figure 11 Diagram of how knee osteotomy corrects joint alignment.34
This image shows an anatomical depiction of pre-osteotomy alignment and post-osteotomy corrected alignment.
Partial or total joint replacement surgery (arthroplasty) removes the damaged joint surfaces and replaces them with plastic or metal parts.1,33 As shown by Figures 12 & 13, this can occur in the knee, hip, fingers, wrists, shoulders, etc.35-37 Figures 14 & 15 depict the spine equivalent, which are artificial disc replacements.38,39 Partial replacement will only remove some parts of the joint and total replacement will replace the entire joint with an artificial joint. The artificial joints can be composed of metal alloys (i.e. titanium, cobalt chrome, or stainless steel), polyethylene, or ceramic.40 Potential complications of arthroplasty include blood clots, internal infections, nerve damage, blood vessel damage, scar tissue, or reduced range of motion. The implant may also experience loosening or wear down over time, which may make it unsuitable for younger patients.37
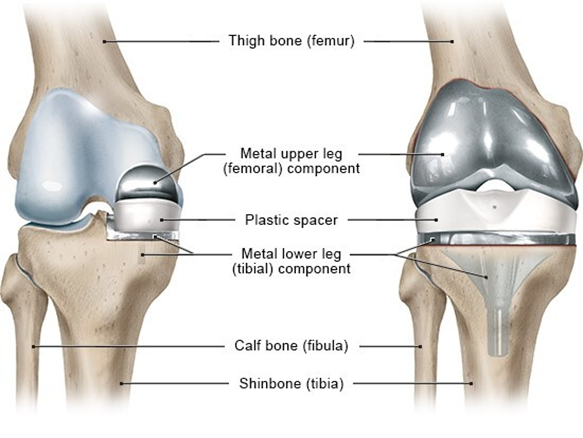
Figure 12 Diagram of partial knee replacement (left) and total knee replacement (right).35
This image shows an anatomical view of the two different types of knee replacements: partial and total.
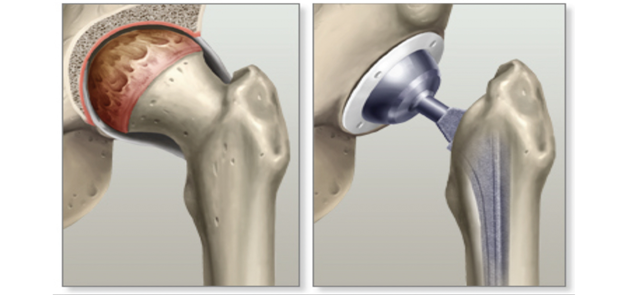
Figure 13 Diagram of hip with osteoarthritis (left) and artificial hip replacement (right).36
This image shows a depiction of a hip joint with osteoarthritis (left) and the resulting total hip replacement (right).
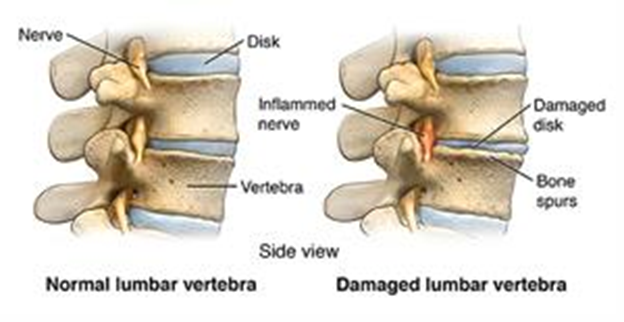
Figure 14 Normal (left) and damaged lumbar vertebrae (right) with degraded disk and bone spurs.38
This image shows a normal lumbar vertebra (left) and a damaged lumbar vertebrae due to inflamed nerves, bone spurs, and damaged disk.
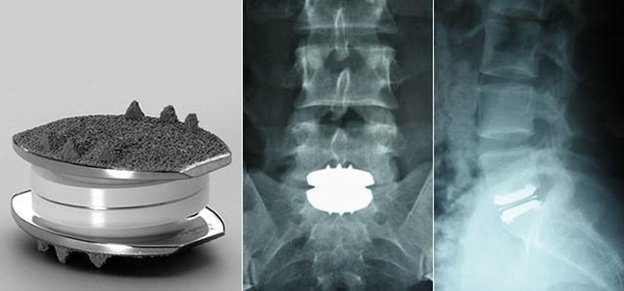
Figure 15 X-ray of an artificial lumbar disc replacement.39
This image shows an artificial lumbar disc replacement and an X-ray of the disc replacement once implanted into the spine.
Surgical options are the most costly and physically taxing. A knee osteotomy procedure typically costs around $20,000. Total and partial knee arthroplasties cost around $25,000 and hip arthroplasties cost around $20,000.41,42 Not only are these procedures expensive, the recovery time and process is much more extensive. Thus, surgery is often used as a last resort.2
There are numerous products targeting osteoarthritis that are in clinical trials from new drugs to therapeutic methods to tissue engineering cartilage. This review will focus on the application of a new drug and tissue engineering cartilage. The new drug, Adelmidrol, is expected to yield more effective treatment when combined with the hyaluronic acid injection.6 Another product in clinical trials is tissue engineered cartilage for patellofemoral osteoarthritis to deliver more effective and long term treatment.5
Hyaluronic acid and adelmidrol (Hyadrol®)
It has been studied that overactivation of mast cells causes chronic neuroinflammation contributing to the development of OA. Thus, the novel approach of combining Adelmidrol with hyaluronic acid emerges. The combination could present an effective treatment in controlling the neuroinflammation process.6
The purpose of the clinical trial is to evaluate the safety and efficacy in the hip (coxarthrosis) and trapezium-metacarpal (rhizarthrosis). To be eligible for this study, the patients must be at least 40 years old, diagnosed with stage II or III coxarthrosis or rhizarthrosis, and have a pain intensity of at least 5 on the Numeric Rating Scale (NRS). The trials are performed as non-randomized, single group assignments. Patients with coxarthrosis received 3 intra-articular infiltrations administered once a week with 2 ml of Hyaluronic Acid and Adelmidrol via sterile single-use device for injection. Patients with rhizarthrosis followed a similar protocol but the injections only had 1 ml Hyaluronic Acid and Adelmidrol. The effectiveness of the injections would be quantified through Western Ontario and McMaster Universities (WOMAC) for coxarthrosis, Disabilities of the Arm, Shoulder, and Hand (DASH) for rhizarthrosis, 11-point NRS, 12-item short form health survey (SF-12), and overall self-assessed health status. Many of these are self-reported questionnaires that ask the patients to assess the degree of their symptoms such as pain, joint stiffness, functional limitations, or general health. The questionnaires are presented at various time points throughout the trial such as after the first, second, and third injection as well as, 1 and 4 weeks after. The safety and tolerability of the injection was assessed based on the incidence and severity of adverse events, these were analyzed at the same time points as the questionnaires.6
This clinical trial is still in the stage of recruiting patients, meaning there are no results thus far. The trial began in July 2024 and is estimated to be complete in July 2025. Overall, the trial aims to understand the effect of the hyaluronic acid and Adelmidrol intra-articular treatment in osteoarthritis patients for pain, joint stiffness, and functional limitations.
Engineered cartilage
This phase II trial of the clinical study aims to assess the efficacy of treating patellofemoral (knee) osteoarthritis using tissue engineered cartilage.5 As mentioned previously, knee OA is the most common form of OA and studies show that it originates in the patello-femoral compartment of the knee (PFOA). Therefore, it is crucial to reduce the progression of OA by targeting the PFOA.
The tissue engineered cartilage is derived from autologous nasal chondrocytes that are cultured in collagen type I/III membrane for 2 weeks to produce an extracellular matrix (ECM) containing cartilage specific proteins. The study compares the autologous nasal chondrocyte tissue engineered cartilage (N-TEC) with the current standard therapy of platelet rich plasma (PRP) injections.
Study participants are selected based on several criteria such as meeting baseline scores on different classification or survey methods and have free range of motion in the impacted knee joint. The study is randomized, parallel assignment with an experimental and control group. The experimental group receives the autologous nasal chondrocyte and ECM proteins. The control group will receive the platelet rich plasma injections. The experimental group will have the tissue engineered cartilage graft implanted into the patellofemoral joint and assessed for 24 months. The control group receives 3 injections of 5 ml each of PRP, autologous Conditioned Plasma ACP®, and Arthrex, spread out such that the patient receives one injection per week for 3 consecutive weeks.6
The primary outcome measure is the mean change in the knee osteoarthritis outcome score (KOOS), a higher KOOS score indicates less knee issues. The change is measured at 24 months and the study is anticipating an increase in KOOS score for the N-TEC. Secondary outcome measures include KOOS subscales, Kujala Anterior Knee Pain Scale, Western Ontario and McMaster Universities Osteoarthritis score (WOMAC), Marx Activity Rating Scale (MARS), and EQ-5d assessment. These measures are assessed at 6, 12, and 24 months, then compared with the baseline and between groups. Trial safety is measured by occurrence of adverse events.
This trial is currently recruiting participants, hoping to enroll 75 patients. The trial began in June 2024 and is anticipated to conclude in September 2029. To summarize, this trial aims to assess the efficacy of tissue engineered cartilage, in hopes of improving the conditions of patients with knee osteoarthritis.43
Osteoarthritis is a chronic, degenerative disease that affects the joints through the degradation of cartilage.1 As risk factors of OA continue to rise, there is an expected increase in consumerism of remedies and therapeutics. The data suggests that there is an increased susceptibility of being diagnosed with OA for elderly women.4 As the demand for therapies increases, various solutions are being considered to provide long term relief to patients. Currently, there is no cure for osteoarthritis but there are various ways to alleviate the symptoms. Possible treatments include invasive and non-invasive options: NSAIDs, injections, surgery, maintaining a healthy weight, exercise, or physical therapy.3 Due to the demand for novel, innovative treatments for OA, such as new drugs are being tested for safety and efficacy along with tissue engineering. These emerging treatments offer much potential in delivering personalized, impactful, and long-term solutions for OA patients beyond the capabilities of those treatments aforementioned.
There is no funding to report for this review.
Helen Ran acknowledges Professor Bill Tawil for guiding the framework of this review, as well as his insightful lectures on biomaterials and tissue engineering, which played a key role in shaping this paper.
The authors declare that there are no conflicts of interest.

©2025 Helen, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.