Journal of
eISSN: 2373-6445


Research Article Volume 15 Issue 6
1Department of Child Development, California State University, USA
2Department of Psychology, University of California, USA
Correspondence: Kaitlyn Breiner, California State University, Dominguez Hills, 1000 E Victoria St, Carson, CA, United States of America, Tel 310-243-2059
Received: November 06, 2024 | Published: December 11, 2024
Citation: Breiner K, Galván A. Neural correlates of social feedback from friends and self-reported happiness in adolescence. J Psychol Clin Psychiatry. 2024;15(6):315-323. DOI: 10.15406/jpcpy.2024.15.00798
Adolescence marks a period of development characterized by increased time spent with friends and learning how to navigate social relationships from feedback received from peers. Research suggests that frontolimbic circuitry, which undergoes significant development during adolescence, helps adolescents learn by encoding feedback. To test this theory, previous studies have given adolescents feedback via someone unknown to them. Understanding how adolescents respond when receiving social feedback from a friend is critical as they engage with friends to help form opinions of themselves. For this reason, we created an ecologically valid task asking adolescents questions about their friendships and showing them responses purportedly provided by their friend.
This study used functional magnetic resonance imaging (fMRI) and a novel Friendship Feedback Task programmed using E-Prime 2.0 to leverage real-life social expectations between 26 adolescent participants (53.8% girls, ages 14-17, M= 15.81, SD= 1.02) from the USA. Participants were presented with positive or negative statements about their friendships with another adolescent while undergoing an fMRI scan. Following the scan, participants were asked how happy they felt when they received social feedback about their friendship.
Results: Adolescents reported feeling happier when social expectations were met compared to when they received positive and negative social feedback. Regions of Interest (ROI) analyses revealed increased ventral striatum activation as expectations were met, greater ventral striatum activation during positive versus negative social feedback; and greater insula and subgenual anterior cingulate cortex activation during negative versus positive social feedback.
These results suggest positive and expected social information engages frontolimbic circuitry previously implicated in reward, while unexpected social information elicits neural circuitry previously implicated in non-social learning in adolescents. This study demonstrates that during adolescence, friends provide important social information, and when the information received is unexpected, it may disrupt the cognitive harmony a teenager has regarding their friendship.
Keywords: adolescence, fMRI, social feedback, ventral striatum, peers
Adolescence is a pivotal time to garner feedback and social information from peers, who provide insight about the dynamic social landscape they experience. Research examining adolescent response to various types of feedback have found that adolescents demonstrate a heightened response to unexpected compared to expected social1 and non-social feedback.2 This reflects a social prediction error framework, whereby individuals encode their expectation of an event (prediction) and compare it to the unexpected response (error),3,4 which is adaptive for learning new information.5 Adolescents demonstrate a desire for positive social feedback,6 and an aversion to negative social feedback.7,8 While most adolescent peer interactions occur with known peers, studies examining adolescent reactivity to peer feedback6,9–14 and peer evaluation7,8,15 tend to utilize a stranger/confederate as a peer. To our knowledge, few have examined adolescent response to feedback when it is from a close friend.16–19 Exploring how adolescents respond to different types of feedback from a friend is important because adolescents often rely on their friends’ opinions to form perceptions of themselves,20 and learn many social skills necessary for development.
The impact of peers on adolescent reactivity is evinced in neuroimaging research, as adolescents change their behavior17 and recruit reward circuitry21,22 in the presence of their peers. It is likely that the observed hypersensitive response in mesolimbic circuitry to emotional and rewarding stimuli, coupled with the protracted development of cognitive control circuitry in the brain, may explain the enhanced sensitivity to social information during adolescence,23 as adolescents rely on peer relationships to help form their identity,24 to fit in25 and to avoid rejection,26 all of which is key in emotion regulation development.27
Functional Magnetic Resonance Imaging (fMRI) research has identified the striatum (a reward sensitive brain region) as a key hub in feedback and learning,28 along with the amygdala,29 insula,30 and anterior cingulate cortex (ACC).31,32 These regions also exhibit significant neurodevelopment during adolescence.33 Indeed, a study examining the neurobiology of feedback in adolescents found that teenagers exhibit a strong striatal response for unexpected positive feedback.2 This is consistent with the heightened striatal response adolescents demonstrate when presented with reward34 and when learning from feedback.35,36 Regions such as the ACC and insula are recruited increasingly with age, suggesting maturation of these regions continues beyond adolescence,37–40 and their recruitment is critical for socioemotional cognitive control.40
Given the amplified response adolescents demonstrate to social feedback and the effect of friendships on self-reported happiness and affect,41–43 the current study adopted a social feedback framework to examine self-report and neurobiological responses to social feedback from a friend. The goal of this study was to identify whether the self-reported and neural mechanisms typically found to be associated with experiencing feedback were also associated with receiving social information from a friend using a novel social feedback task in adolescents. This is of particular importance, as research has found that feedback and validation (e.g., amassing online followers,44 or receiving “likes” on social media accounts)45 is predictive of mental health and well-being. Examining the nuance of social feedback/validation (e.g., whether it is positive, negative, expected) and its gradation of differing degrees of unexpectedness both neurobiologically and via self-report may elucidate whether one type of feedback is more problematic than another, as it pertains to adolescent mental health. Previous studies have utilized paradigms whereby participants received social feedback from a stranger, or non-social feedback (e.g., expectations about task performance from a friend),17 but not social feedback (information the target would arguably be concerned about outside of the laboratory) from a friend. Because providing real-time feedback is challenging in a laboratory setting, we implemented an ecologically valid approach by leveraging real-life social expectations between friends and manipulated them in the laboratory.
We hypothesized that adolescents would: 1) report feeling happier as social feedback transitioned from negative to expected, to positive.6–8,46 Considering literature citing friendship predicts happiness43,47 and well-being,48 we hypothesized that 2) adolescents would report feeling happier learning their friend reported something better than they expected (e.g., how nice they are) compared to something worse than they expected; and 3) activate brain regions previously associated with feedback and learning, such as the ventral striatum (VS)2,6 when they learned something better than expected from their friend compared to worse than expected. Adolescents have recruited the ACC when processing negative social feedback,13 a region that is also recruited when processing negative emotions.49 Similarly, researchers have found the insula to be implicated when processing negative emotion and social feedback.50,51 Thus, we hypothesized 4) increased activation in the ACC and insula when adolescents received negative compared to positive social feedback from their friend.
Participants
Twenty-six right-handed adolescent participants (53.8% girls, ages 14-17, M= 15.81, SD= 1.02) and their age- (M= 15.56, SD= 1.19) and gender-matched friends were recruited from the Los Angeles area to participate in the study. Participants were informed they would be answering questions about their friendship. Target participants were recruited to participate in two study sessions, while friends were recruited to complete one session. The target participant group was unique from the friend group (target participants did not also serve in the friend group), and was ethnically diverse (34.6% White, 30.8% Hispanic/Latino, 11.5% African American, 7.7% Asian, 15.4% Other), and did not differ from the friend group by race/ethnicity (χ2(4, N = 26) = 7.46, p = 0.11) or socioeconomic status—measured as average level of parental education obtained in the household (χ2(10, N = 26) = 13.23, p = 0.15).
ProcedureEach target participant was recruited as part of a larger study, and brought a same-aged, same-gender close friend to the first of two sessions. At the first session, participants and their parents were informed of the study’s procedures orally and were presented with hard copies of assent/consent forms, (which also described the study procedures) to sign. We obtained written assent from participants and written consent from their parents/guardians in accordance with ethical standards and guidelines from the UCLA Medical Institutional Review Board (IRB) 3, the governing board that approved this study. During the first session, the target participants and their friends provided demographic information and completed a Friendship Questionnaire. The session took approximately 20 minutes and both participants received $10.00 compensation for participation.
Target participants were invited to the Staglin Center for Cognitive Neuroscience between 7 and 14 days after the initial session to participate in the second session and undergo an fMRI scan. Most subjects returned within this time frame, though due to scheduling constraints, 84% of the participants returned within three weeks. All but one subject (who moved away but returned 111 days after the initial session) returned within the month (M= 16.46, SD= 20.17). While in the scanner, target participants completed the Friendship Feedback Task. Upon completion, they exited the scanner and answered a post-task survey, which reminded them of the questions they saw in the scanner, their expectations, and their friend’s responses. They reported how happy they felt after viewing their friend’s response to each item. At the end of the second session, the target was debriefed and told that the items presented during the Friendship Feedback Task were manipulated by the research team. Participants were asked whether they believed the manipulation, one participant reported feeling dubious to a few items, though that individual’s responses were not statistically different from other participants’. Following debriefing, participants were asked if they had any questions about the study, were paid $45, and thanked for their time.
Materials and apparatus
Session 1
The target participant and the friend completed the questionnaires in separate rooms. They completed a demographic questionnaire (including age, gender, race, parental education, and friendship duration (in months)), and a Friendship Questionnaire, comprised of 40 items, which were adapted from the McGill Friendship Questionnaire, Friendship Functions,52 and the Friendship Quality Questionnaire.53 Items from the questionnaires were chosen at random and adapted for administration to adolescents. Thirty-five of the questions were social in nature, five were non-social. Social items were evaluative and required the participant to consider how their friend considered their friendship. Sample social items for the target included: “My friend is nice;” “Our friendship is accepted by my friend’s family.” The remaining questions asked about non-social information and required the participant to consider factual information about their friend (e.g., “My friend likes horses;” “My friend hates country music”). Participants were asked to predict their friend’s responses to the items on the questionnaire, on a scale of 1 (not true at all) to 10 (definitely true). Only the target participant’s responses were kept for the study. Certain items were reverse scored to ensure that higher numbers indicated a positive sentiment. Both participants were informed their respective responses on the Friendship Questionnaire would be confidential and that their answers would not be shared with their friend so as to encourage honest reporting and reduce unintentional inflated responses. Participants were friends for an average of 44.54 months (SE = 8.63), and in general, had positive expectations of their friendship (M = 9.00, SE = 5.81). They were 32.10% accurate in their expectations of their friend’s actual responses to the questionnaire (SE = .033).
Session 2
Participants completed the Friendship Feedback Task while undergoing a brain scan. The goal of this task was to measure neural and affective responses of receiving expected or unexpected feedback from a friend. In this task, we presented target participants with statements that were purportedly based on the friends’ responses to the Friendship Questionnaire. The responses were presented as if they represented the friend’s true response, but in fact they were manipulated. Manipulation of item feedback was pseudo-random—based on target responses from the Friendship Questionnaire, such that we were limited to the extent feedback could be increased/decreased (e.g., expectation of a 10 could not be increased at all)—and we aimed to keep most feedback +/- 5 from the original rating to encourage task believability. Thus, the task was preprogrammed for each participant individually. To create positive feedback, we issued responses to be greater than the target’s expectation (e.g., if the target responded with a “6” that her friend would think she is nice, we issued a greater value, such as a “10”). To meet the expectations of the participant, we issued a response to match the target’s prediction (e.g., if the target responded with a “6”, we issued a “6”). To create negative feedback, we issued a response worse than the target’s expectation (e.g., if the target responded with a “6”, we issued a lower value, such as a “2”).
Positive and negative feedback was manipulated based first on a wholistic approach, then at an individual value approach. The wholistic approach was taken first to consider all responses from the target. For example, if the target expected 90% of the responses to be highly positive (e.g., 8, 9, or 10), we were sensitive not to deviate too far from those scores. Thus, most manipulated feedback that was provided was still generally positive, so the participant did not suspect our manipulation. The individual value for each item was considered next, to ensure we had a range of values (rather than all 1’s) from which we could examine deviations in expectations for a given participant. The 40 items were presented only once. Because the dyads were close friends, participants tended to have relatively positive expectations of their friends’ responses, which limited our ability to change feedback valence (positive or negative) and value (i.e., adding to or subtracting from the number the target predicted) of the issued responses. We also avoided adding too many extreme values so as to keep the manipulation believable, and removed values +/- 8 and 9, as they reflected only 1.6% of analyzable trials, and may have invoked feelings of confusion (especially if they were negative). Thus, during the session, each participant was presented with an average of 14.96 positive items, 7.04 items where expectations were met, and 17.88 negative items. Due to concerns of lack of inter-individual variability, feedback was experimenter-manipulated to distribute variability across values. The total number of trials for each feedback valence and value are provided in Table 1.
|
Valence |
Negative |
Met |
Positive |
||||||||||||
|
Value |
-7 |
-6 |
-5 |
-4 |
-3 |
-2 |
-1 |
0 |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
|
All Trials (1001) |
17 |
26 |
35 |
62 |
90 |
101 |
120 |
183 |
127 |
90 |
63 |
43 |
31 |
13 |
9 |
|
Analyzed Trials (939) |
15 |
23 |
34 |
57 |
85 |
96 |
113 |
172 |
124 |
84 |
59 |
39 |
28 |
10 |
8 |
|
Social Trials (800) |
12 |
21 |
27 |
47 |
71 |
79 |
96 |
146 |
113 |
74 |
49 |
32 |
24 |
9 |
5 |
Table 1 Number of trials based on valence and value for all 26 participants
The Friendship Feedback Task was programmed in E-Prime 2.0 and was presented through an LCD Optoma projector connected via fiber optic cables. Participants were presented with all 40 statements from the Friendship Questionnaire and were asked to press a button on a 4-button button box with their right index finger to proceed to the next question/trial. The trial began with the presentation of the question (2000ms), followed by the target’s expectation (4000ms), a 2000-6000ms jittered inter-stimulus-interval (ISI), the experimenter-manipulated issued response (4000ms), a jittered ISI (2000-6000ms), and a request to press a button to proceed to the next trial (event/maximum 5000ms allotted time), followed by a 4000-8000ms jittered inter-trial-interval (ITI). Participants were asked to press the button so we could ensure they were paying attention to the screen, before a jittered fixation cross appeared on the screen, and the subsequent trial began (Figure 1).
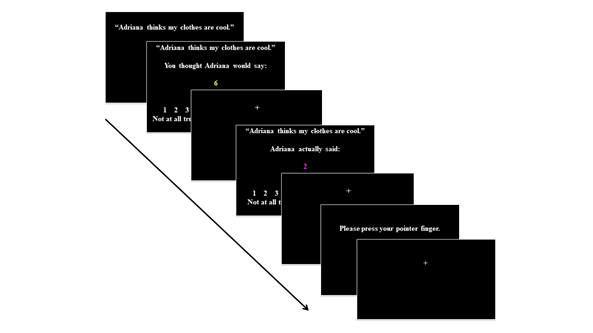
Figure 1 Friendship feedback task.
While in the MRI scanner, participants viewed information pertaining to their social expectations and the manipulated outcome before pressing their pointer finger to move on to the next trial.
Following the scan, participants completed a questionnaire that contained each statement from the original Friendship Questionnaire, along with their predictions and their friend’s “feedback.” For example, participants were reminded of the statement, “My friend thinks I’m nice” and were shown that they expected their friend to report 6, while their friend “reported” (experimenters manipulated the response to be) 10. They were asked to report how seeing this made them feel on a scale of 1 (very hurt) to 10 (very happy). Participants rated their feelings outside of the scanner so we could assess how they reported feeling when they reviewed what they expected alongside the manipulated response. We did not do this in the scanner due to time/financial restrictions. Following the study, participants were debriefed and asked about the believability of the design. One participant reported feeling somewhat dubious of a few positive feedback items, 25 believed the manipulation. None of the participants commented on feeling betrayed or confused by the confidentiality assurance that was made in Session 1, and instead expressed enthusiasm about their experience.
Design
The present study was a within-subjects, event related design. Our independent variable was the presented feedback, operationalized by modifying a presented response to be better than, equal to, or worse than the target’s expectation. Our dependent variables were 1) self-reported response (as indicated on the post-scan questionnaire), and 2) neural activation when targets viewed the manipulated feedback. Neural activation was examined using a priori regions of interest (ROIs).
Behavioral analyses
IBM SPSS Statistics Software, version 23.0 was used to analyze self-reported responses to the post scan questionnaire. The data were analyzed to determine: 1) effects of valence (whether the social feedback was negative, positive, or whether social expectations were met); and 2) parametric effects of incremental differences of the social feedback—which was represented by feedback that ranged between 7 values below the target’s expectation (FB-7) and 7 values greater than their expectation (FB+7).
fMRI analyses
Images were collected using a 3-Tesla Siemens Trio MRI machine equipped with a 16-channel head coil at the Staglin Center for Cognitive Neuroscience at UCLA. Two structural MRI images were collected at the start of the scan: a T1-weighted magnetization-prepared rapid-acquisition gradient echo image (MPRAGE; 160 sagittal slices; slice thickness, 1 mm; repetition time (TR), 2000 ms; echo time (TE), 2100 ms; matrix, 256 x 256; and field of view, 250 mm) and a T2-weighted matched bandwidth high-resolution scan were acquired for registration. One functional run was collected and consisted of 440 T2*-weighted echo-planar images (EPIs) (34 slices; slice thickness, 4 mm collected sequentially on the oblique axial plane; TR, 2000 ms; TE, 30ms; flip angle, 90°; matrix, 64 x 64; and field of view, 192 mm). The first three TRs of the run were automatically discarded by the scanner.
Data preprocessing and analyses were conducted using the FMRIB (functional magnetic resonance imaging of the brain) Software Library (FSL) version 5.0 (www.fsl.fmrib.ox.ac.uk). Images were corrected for motion using MCFLIRT (motion correction, using FMRIB’s linear image registration tool) and de-noised using multivariate exploratory linear optimized decomposition into independent components analysis. Data were smoothed using a 5-mm full-width-half-maximum Gaussian kernel and filtered with a nonlinear high-pass filter (66-s cutoff). A three-step registration process was used to align individual participant data into standard Montreal Neurological Institute (MNI) space. EPI images were first registered to the matched-bandwidth image, then to the MPRAGE image, and finally to MNI space. Two subjects’ data required truncating of the last 115 volumes due to excess absolute motion (> 2 mm) in the latter half of the scan—their corresponding self-report data was also discarded. Motion for all other participants was less than 2mm in any given direction.
Data were analyzed using a subtraction method to model differences between the target’s expectation and the experimenter presented feedback. A general linear model with regressors for each event of interest was conducted for each participant. Contrasts of interest were first performed at the subject level based on valence (positive feedback > baseline, expected feedback > baseline, negative feedback > baseline). Neuroimaging analyses were performed to examine neural response to valence of the information (positive feedback, negative feedback, and when expectations were met). To examine contrasts of interest based on a priori hypotheses, at the group level, we compared positive > negative feedback, and negative > positive feedback on ROIs known to be associated with positive and negative social feedback. This regressor of interest was convolved with a canonical hemodynamic response function. Six motion parameters were included as covariates in the model for each run for each participant.
Each participant’s data were modeled using a three-column regressor that contained the onset of each event (either the expectation or feedback—depending on the contrast of interest), its duration, and a standard weight of 1 (to assess each event equally for non-parametric effects); or a weight that reflected the iterative value of the event (e.g., -2 for a feedback value of -2) to model parametric effects.
Analyses were conducted using FMRI expert analysis tool (FEAT), first at a lower level to represent one level of a condition within an individual subject, then at a second level using a fixed-effects model to represent all levels of a condition for one subject. Finally, data were analyzed at a group level analysis using FMRIB’s Local Analysis of Mixed Effects with automatic outlier detection to group all participants together and compare contrasts of interest. Functional ROI masks containing a cluster radius of 6 voxels (2x2x2) were created for the amygdala, dorsal anterior cingulate cortex (dACC), insula, subgenual anterior cingulate cortex (subACC), and ventral striatum (VS) based on functional probability maps from Neurosynth.54 These masks were added as the Pre-threshold Masks for each respective ROI and were clustered at the voxel level with a Z threshold of 2.3 and probability threshold of p = .05, corrected. We assessed activated clusters within the ROIs by performing a small volume correction analysis.
Self-report questionnaire
To determine how participants felt when their social expectations were met compared to when they received positive and negative social feedback, one-way repeated measures ANOVA was performed. This analysis revealed a significant difference between positive, negative, and expected social feedback (M = 9.51, SE = 0.12) F(2,50) = 96.71, p < 0.001 such that participants reported feeling happiest when social expectations were met compared to when they received positive t(25) = 5.69, p < .001 and negative social feedback t(25) = 12.47, p < 0.001 (Figure 2). To determine whether participants reported feeling happier upon experiencing positive compared to negative social feedback, a post-hoc paired-samples t-test was performed. Our second hypothesis was supported, such that participants reported feeling happier when they received positive social feedback (M = 8.23, SE = 0.25) compared to negative social feedback (M = 5.56, SE = 0.26) t(25) = 8.41, p < 0.001.
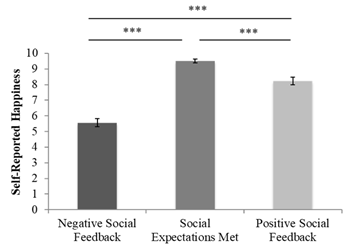
Figure 2 Self-reported happiness following the friendship feedback task by valence.
Participants were happiest when their social expectations were met compared to when they received negative or positive social feedback.
To further unpack these results, a multilevel regression using a random intercept of a subject was performed to determine whether there were differences in self-reported happiness parametrically based on iterative FB values. Iterative FB values represented the magnitude with which feedback occurred (e.g. a -7 is greater negative social feedback than a -1). Because the plotted data reflected a curvilinear relationship consistent with a quadradic trend, we tested it for a quadratic trend and found our first hypothesis was not supported. We found a significant quadratic relationship, such that participants reported feeling least happy when they received negative social feedback, reported feeling increasingly happier as social expectations were met, but reported feeling less happy as social feedback was increasingly positive F(2,802) = 221.67, p < .001 (Figure 3).
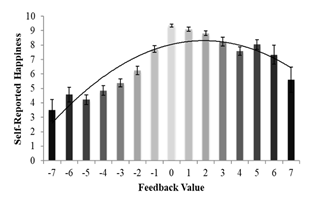
Figure 3 Self-reported happiness following the friendship feedback task by value.
Participants were increasingly happier when their expectations were met and were less happy the more unexpected the social feedback became.
fMRI analyses
fMRI analyses were conducted on a priori regions of interest (ROIs) implicated in processing social and emotional information, including the amygdala,55 dorsal anterior cingulate cortex (dACC), insula,56 subgenual anterior cingulate cortex (subACC),57 and VS.2 We examined whether differences were present between negative and positive social feedback. Our third hypothesis was supported, such that for positive compared to negative social feedback, participants recruited the VS (MNI coordinates: 8 x 8 x -6; Z = 2.62, p < .05). Our fourth hypothesis was supported, such that for negative compared to positive social feedback, participants recruited the insula (MNI coordinates: 40 x 22 x -4; Z = 2.40, p < .05) and subACC (MNI coordinates: 0 x 24 x -8; Z = 2.55, p < .05) (Figure 4).
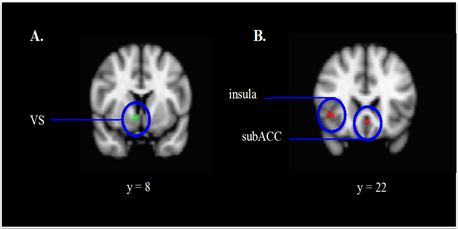
Figure 4 ROI contrasts.
Participants activated the ventral striatum upon receiving positive social feedback and activated the insula and subgenual anterior cingulate cortex upon receiving negative social feedback.
In accordance with the behavioral analyses, we modeled parametric effects on the ROIs to examine neural activation based on the valence and value of the feedback. Because the plotted data reflected a curvilinear relationship consistent with a cubic trend, we tested it accordingly. These results revealed a significant cubic trend in the dACC (F(3,11)= 4.86, p = .022; R2 = .570) and amygdala (F(3,11)= 8.83, p = .003; R2 = .707), such that activation in these regions increased when iterative feedback was moderately positive (e.g., +2), and decreased when iterative feedback was moderately negative (e.g., -5) and very positive (e.g., +6). A significant quadratic trend was revealed for the VS (F(2,12)= 6.06, p = .015; R2 = .502) such that activation in this region increased most when expectations were met or were slightly positive (e.g., 0, +1) and decreased when feedback was very unexpected (e.g., -/+7) (Figure 5). A cubic trend was tested against the VS activation and was not significant. We did not find a significant parametric trend in recruitment of the insula or subACC across iterative FB values.
The goal of this study was to examine whether self-report and neural correlates associated with feedback were also implicated in experiencing seemingly real social feedback from a friend in adolescents by using a novel, ecologically valid task. Adolescents’ self-reported happiness increased as the social feedback transitioned from negative to expected and positive. Notably, happiness was greatest when social expectations were met. Neurobiologically, participants recruited the VS in a pattern that resembled their self-reported happiness to receiving expected and somewhat positive social feedback. Participants also demonstrated greater recruitment of the VS for positive to negative social feedback; and greater recruitment of the subACC and insula for negative compared to positive social feedback.
We enrolled a friend in the study to enhance believability of the study, to ensure feedback appeared real to the target, and to evoke a strong response due to the nature of the task (requiring feedback from an actual friend). Enrolling an unknown/unseen peer for this study may have yielded different results, such that adolescents may not have believed or cared about the feedback they received—as the feedback was specific to knowledge about the participant. However, it remains unknown whether the self-reported or neurobiological responses would have been different or diminished if another known person (e.g., a sibling), or an unknown peer/confederate completed the task in place of the friend; as to our knowledge, no other study has compared adolescent response to social feedback from a friend, an unknown peer, and a recording from a confederate. Various studies of peer feedback/presence over the past 15 years have implemented one of the aforementioned “peers” (see studies on preference,58,59 risk-taking,17,21,60 choice,61,62 and affect11,44 as examples). Future research should consider enrolling a friend and an unknown peer to compare whether the presence of either yields a differential response.
Participants reported feeling happier upon receiving positive social feedback compared to negative social feedback regarding their friendship. Parametric analyses revealed additional differences in self-reported happiness, such that participants were happiest to learn they were accurate in their social prediction or to receive slightly positive feedback compared to larger social feedback magnitudes. Our first hypothesis (that adolescents would be happier to receive positive social feedback compared to receiving expected social feedback) was not supported. This result was unexpected and is a novel contribution to the literature. We posit that in contrast to other feedback studies,10,63 greater positive feedback was not reflective of increased reward; instead, unexpected social feedback may have been either more surprising or indicative of decreased knowledge of friendship, which may have resulted in feelings of decreased happiness/affect when feedback was positive or negative—as positive feedback may not have felt complimentary and instead, represented a prediction error.16 Reminding participants of their predictions and their friends’ responses may have felt threatening64,65 whereby participants knew their friend could have reported something worse than they expected. In turn, this may have amplified the happiness they reported when expectations were met, perhaps indicating they were relieved65,66 and were pleased to experience reciprocity67 or something better than expected from a friend.47,48 It is also plausible that because the adolescents in our sample tended to be friends for a little under two years, they felt more connected when their expectations were met, suggesting increased positive emotions and trust were evoked upon receiving validation from their friend.68 We suggest that experiencing unexpected social feedback of any kind is conflicting69 and emotionally arousing for an adolescent, as they are hyper-sensitive to peer feedback, and learning they are incorrect about their friendship (even if the feedback is positive51) may be disconcerting compared to learning they are correct.
Neuroimaging results revealed that adolescents recruit the VS, insula, and subACC differentially when they experienced positive compared to negative social feedback. We posit that the increased happiness participants reported for positive compared to negative social feedback suggests participants were happier and recruited the VS (a region that has previously been implicated in reward receipt)34 when they experienced positive social feedback compared to negative social feedback. When they experienced negative social feedback, they reported feeling less happy by comparison and recruited the subACC (a region implicated in rejection,13 particularly in youth)70 and the insula (a region implicated in social pain).56 These ROI analyses demonstrated significant differences comparing positive to negative valences, but not when feedback was expected.
To further probe these differences and compare them to self-reported affect, parametric analyses were conducted on the ROIs and revealed adolescents recruited the VS (and to some extent, the dACC and amygdala) in a pattern reflecting self-reported affect. The VS was recruited more when feedback was expected, and less so the more unexpected the feedback became. Given the role of the VS in learning, reward, and feedback, it could be possible that initially, recruitment was greater for more unexpected trials to reflect a significant prediction error signal,2,71 and that eventually, the VS was recruited more when feedback was expected because it was more rewarding, as it was consistent with participants’ expectations.72,73 It is likely that upon receiving expected feedback in real-time, adolescents recruited regions implicated in error-detection and monitoring (such as the dorsal ACC and anterior insula), as well as emotional processing (e.g., the amygdala) over and above reward regions, perhaps because they were contemplating the nature of the accuracy of the feedback (e.g., if it confirmed positive or negative information about their friendship).74 The parametric pattern of recruitment of the amygdala and dACC may be indicative of the emotional salience of the feedback when it was more plausible (moderate iterative value) and positive,75 especially when considering the functional connectivity of these regions to the VS.76 These results are consistent with recent work demonstrating a similar pattern of activation in these regions when irritable adolescents receive unexpected feedback that is nice or mean.77
Recent fMRI studies assessing the subACC and insula suggest that they may also be recruited when considering the self,78 and that a broader network (including the subACC and insula) is recruited when experiencing rejection or learning unexpected information.79 While we hesitate to draw reverse inferences, we posit adolescents in our study recruited these aforementioned regions upon learning information that challenged their understanding of their friendship (e.g., realizing their expectation of their friend’s response was incorrect), while simultaneously learning their friend thought something negative about them (e.g., not being as smart as they expected).
While adolescents reported after the scan that they felt happier to learn they were accurate about their friendship, it is plausible that they did not feel that way at the time in the scanner when they saw the same items—perhaps due to the anticipation that built prior to viewing the feedback. These results highlight important distinctions and associations in how adolescents respond neurobiologically and report feeling when they learn new (potentially important) social information from someone they care to receive that feedback from.
While our study has notable strengths (namely, its ecologically valid design and results demonstrating similar patterns of self-reported happiness with VS activation in response to social feedback), we acknowledge its caveats. We were limited in the extent to which we could modify the valence and value of feedback. Thus, it was more challenging to assess positive social feedback (many participants had relatively high expectations) compared to negative social feedback. Future research in this area should consider how to increase values of positive social feedback to test the behavioral and neurobiological effects more precisely. Non-social items were developed to compare to social items. Analyses comparing them revealed no significant differences, perhaps because there were few non-social items, and the items described attributes of the target. Future research should focus on a truly non-social iteration with an equal number of trials for a more precise comparison. When asked about task believability, one participant questioned the believability of a few of the items (specifically, two that were more positive than expected); however, this participant’s data did not statistically differ from other participants, and task believability was not associated with friendship quality or duration. We recognize that there was not an equal distribution of feedback values across trials due to natural variability between subjects. When we accounted for this in our analyses, and compared it to our planned analyses, we found no significant differences. Additionally, we posit that responses of a wide range (such as FB-7 to FB+7) to social feedback in a friendship may encompass thought processes (e.g., confusion) that a restricted range may avoid. We acknowledge that more unexpected feedback likely elicited responses that were indicative of confusion and may have sewn doubt of the veracity of the task. We were careful in distributing feedback that reflected various valences and magnitudes and issued more that were closer to expected. If we equally distributed feedback across valence/value, we expect participants would doubt the feedback presented in the task, and inferences made about the behavioral and neurobiological results may instead consider participant surprise, confusion, and perhaps concern/anxiety—as participants would very frequently learn their expectations about their friendship were incorrect. We recognize that by restricting our analyses, we also limit the amount of statistical power, and remove any significant responses to feedback that may lend themselves to future responses. Finally, while we were primarily interested in understanding adolescent response to feedback from a friend, we recognize that few conclusions can be drawn to distinguish developmental differences or differences between a friend and a stranger, as we did not assess these effects in an adult or non-friend comparison group. Future research should consider these additional comparisons to determine the behavioral and neurobiological nuances of receiving feedback from a friend as an adolescent.
In sum, this study contributes to the literature by highlighting differences in adolescent responses to social feedback from a friend based on valence and magnitude of feedback. Understanding these differences is important, as differential feedback (in novelty, valence, and value) has been known to influence behavior, especially in adolescence, as teenagers’ behaviors can be reflective of their diminished cognitive control in affective situations. Moreover, most adolescent peer interactions occur with friends, and adolescents are particularly keen on social acceptance. Thus, by incorporating “authentic” feedback, participants are actually interested in outside of the laboratory, we can elucidate differences that may not otherwise appear had we used a confederate or virtual peer. Learning new social information is critical during adolescence, as it is a time in development spent largely with friends. Our study finds that adolescents who are close friends prefer to learn they were correct in their expectations about their friendship, even if their friend reports something better than they expected. Learning unexpected social information at this age can be discordant with internal representations, which perhaps yields differential neurobiological activation based on the magnitude and valence of the expectation difference. We conclude that any new and unexpected social information garnered during this age about a close friendship may be disruptive to the cognitive harmony that a teenager has regarding their friendship, which may add to a growing social knowledge about friendships, validation from peers, and have implications for adolescent behavior in affective contexts.
The authors thank K. Do for her assistance with participant recruitment.
This research project was partially or fully sponsored by (The University of California, Los Angeles Training Program in Translational Neuroscience of Drug Abuse) with grant number (NIDA T32 Grant NPI/NIH T32DA024635).
The authors declare no conflicts of interest.

©2024 Breiner, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.