Journal of
eISSN: 2373-6410


Conceptual Paper Volume 15 Issue 1
Department of Neurology, Universidad de Antioquia, Colombia
Correspondence: Gloria Yuliet Cartagena, Department of Neurology, Neuroclinica, Colombia
Received: March 01, 2025 | Published: March 13, 2025
Citation: Cartagena GY, Mesa DV, Arango JAJ. Clinical reasoning: 63-year-old man with HIV, neuropathy and elevated M protein. J Neurol Stroke. 2025;15(1):15-17. DOI: 10.15406/jnsk.2025.15.00614
A 63-year-old man with a clinical history chronic HIV infection for at least 30 years with HIV-associated neurocognitive disorder (HAND), poor compliance to antiretroviral drugs, and a poor immunovirological control (CD4+ T lymphocytes 97/mm3). He reports several months of progressive decrease in muscle strength in four extremities, predominantly in the lower limbs, with associated numbness and tingling. Physical examination was significant for orbicularis oculi paresis, flexors cephaloparesis, predominantly proximal quadriparesis, absence of patellar and Achillean reflexes, and hypoesthesia for superficial and deep sensation modalities with an asymmetric non-length dependent presentation.
Electromyography and conduction velocity consistent with demyelinating polyneuropathy, and cytological albumin dissociation in CSF; A diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP) associated with HIV was made. Intravenous immunoglobulin (IVIG) was given with a partial improvement. One month later, he presented with worsening of limb weakness, difficulty walking and dysphagia. Serum protein electrophoresis showed a monoclonal gamma peak and serum immunofixation showed a monoclonal IgG/lambda band and a paraproteinemic neuropathy is suspected. Complementary studies are requested to clarify etiology, which indicated hypercalcemia, renal failure, anemia and hepatomegaly, and bone marrow aspiration and flow cytometry were requested (Figure 1).
Questions to consider:
What are the characteristics of HIV-associated neuropathy?
Polyneuropathy (PNP) affects 50% of patients with HIV in the course of the disease. It can compromise the sensory, motor or autonomic function, and is considered to be an indirect effect of inflammation on the nerve that leads to a neuritic degeneration, given that HIV does not directly infect axons or Schwann cells.1 The main risk factors are low CD4 T cell counts and high viral load.
Neuropathy subtypes in patients with HIV
Distal symmetric PNP: the most frequent presentation, representing 35% of cases. Due to axonal damage, it is usually length-dependent and predominantly sensitive with the involvement of small fibers.2 A 10mm deep punch biopsy of the skin, 10 cm above the lateral malleolus is recommended, which has a sensitivity of 90% and specificity of 95% for diagnosis. The samples are immunolabelled against the product of the pan-neuronal marker protein gene; density of intraepidermal nerve fibers should be measured, which is considered pathological when below the 5th percentile according to age and gender.1,3
PNP secondary to toxicity by antiretroviral therapy (ART): mainly occurs as a result of nucleoside analog use, such as stavudine, didanosine, and zalcitabine. These agents generate neuropathy through neurotoxicity due to mitochondrial DNA depletion, by inhibiting the gamma-DNA polymerase used for mitochondrial replication. Elevated levels of lactate have been described at the cellular level in patients with ARV neuropathy with a correlation between increased lactic acid and inhibition of mtDNA. All of this leads to inflammatory damage to sensory axons and the dorsal root ganglia.1
Demyelinating inflammatory polyneuroradiculopathy: it can be acute or chronic. Acute inflammatory demyelinating polyneuroradiculopathy (AIDP) may occur in early stages of HIV as a monophasic condition where patients develop rapidly progressive weakness and areflexia within 4 weeks. In contrast, chronic inflammatory demyelinating polyneuroradiculopathy (CIDP), presents after 8 weeks with intermittent proximal or distal weakness, and generally occurs later in the course of HIV. For both, EMG/CV can show demyelinating neuropathy, with prolonged latency and conduction blocks and CSF analysis may show cytological albumin dissociation (increased protein> 100mg / dl and <50 leukocytes / μl).1,4
Questions to consider
Is monoclonal gammopathy the cause of neuropathies in all patients with an elevated M protein?
What complementary studies help to determine the diagnosis?
The term paraproteinaemic neuropathy is used to refer to a heterogeneous group of neuropathies that have as a common characteristic an excessive amount of abnormal immunoglobulins that are usually monoclonal and are known as M protein. These explain 10% of all PNPs, however, they occur in 1% of the healthy general population, increasing to 3% in those over 50 years (5). Within the spectrum we found: monoclonal gammopathy of undetermined significance (MGUS), the most common, generally subclinical and benign in its course. It can manifest as an acquired distal symmetric demyelinating neuropathy with M protein and less frequently as chronic ataxic syndrome with ophthalmoplegia, M protein, cold agglutinins, and antidisialosyl antibodies (CANOMAD).6 MGUS is a premalignant clonal disease, with a risk of progression to multiple myeloma or other plasma cell neoplasia of about 1-2% per year. It is classified according to the type of monoclonal protein into non-MGUS IgM, MGUS IgM, and light-chain MGUS. Waldenstrom's macroglobulinemia is characterized by infiltration of the bone marrow by lymphoplasmacytic lymphoma and monoclonal gammopathy of the IgM type in any concentration, and generally manifests clinically with fatigue, organomegaly, and hyperviscosity.7 In turn, POEMS syndrome is an acronym that refers to polyneuropathy, organomegaly, endocrinopathy, M protein and skin change. POEMS syndrome is associated with infiltration of the bone marrow by plasma cells and Castleman's disease in the lymph nodes, the monoclonal component is by lambda light chains in most cases. Peripheral neuropathy, typically demyelinating, is a major criterion for the diagnosis of the disease. AL amyloidosis is another plasma cell neoplasm characterized by the deposition of amyloid fibrils composed by fragments of monoclonal light chains, and may be primary or associated with another plasma cell neoplasm; Clinically, it is characterized by fatigue, organomegaly, macroglossia, heart disease, skin lesions, and autonomic polyneuropathy manifested as orthostatic hypotension, abdominal pain, postprandial vomiting, erectile dysfunction, and neurogenic bladder.8 Lastly, multiple myeloma is diagnosed by the presence of 10% or more plasma cells in the bone marrow, associated with characteristic clinical manifestations such as anemia, kidney failure, hypercalcemia, and lytic bone lesions. In the absence of clinical manifestations, multiple myeloma can be diagnosed by the presence of 60% or more plasma cells in the bone marrow, a light chain ratio greater than 100, or > 1 focal bone lesion by magnetic resonance imaging.6
There are 3 fundamental aspects that guide the etiology: the medical history, the electrodiagnostic findings and the elevated M protein type (Table 1). Approach based on clinical history and physical examination: should be focused on the search for systemic manifestations such as organomegaly, endocrinopathy, skin lesions, weight loss that leads to thinking about POEMS syndrome; if there are constitutional symptoms and autonomic symptoms, it is suggestive of a primary light chain amyloidosis.6
|
Hematological disorder
|
Clinical presentation
|
Monoclonal protein
|
Peripheral neuropathy phenotype
|
Electrodiagnostic phenotype
|
|
Monoclonal gammopathy of undetermined significance (IgM-MGUS)
|
Subclinical DADS-M Conversion to hematologic disorder 1% |
IgM kappa
|
Distal long-fiber sensory neuropathy with ataxia
|
Demyelinating, excessively prolonged distal latencies, absence of sural response, marked degrees of temporal dispersion.
|
|
Waldenström Macroglobulinemia
|
Fatigue, ataxia, lymphadenopathy, hepatomegaly, splenomegaly
|
IgM kappa
|
Distal long-fiber sensory neuropathy with ataxia
|
Major axonal than demyelinating (with prolonged distal latencies)
|
|
Multiple myeloma
|
Fatiga, lesiones óseas, infecciones recurrentes, síntomas sensitivos
|
IgG more than IgA
|
Sensory, sensory-motor or motor length-dependent neuropathy
|
Generally Axonal
|
|
POEMS syndrome
|
PNP, organomegaly, DM, hypothyroidism, skin lesions |
IgG o IgA, lambda
|
Distal > proximal sensory motor polyradiculoneuropathy |
Demyelinating
|
|
Amiloidosis de cadena ligera
|
Facial purpura, hepatomegaly, macroglossia, dysautonomia symptoms |
Lambda
|
|
Generally Axonal
|
Table 1 Main features of paraproteinemic neuropathies
An approach based on electrodiagnostic studies: demyelinating PNPs mainly suggest MGUS or POEMS syndrome, if, on the contrary, it is axonal it is suggestive of multiple myeloma, Waldenstrom’s macroglobulinemia or primary amyloidosis.6 An approach based on the M protein isotype: when the elevated isotype is IgM, it suggests MGUS or Waldenström's macroglobulinemia, whereas multiple myeloma is characterized by elevation mainly of IgG and to a lesser extent of IgA, and in POEMS syndrome and primary amyloidosis, lambda light chains will predominate (Figure 2).
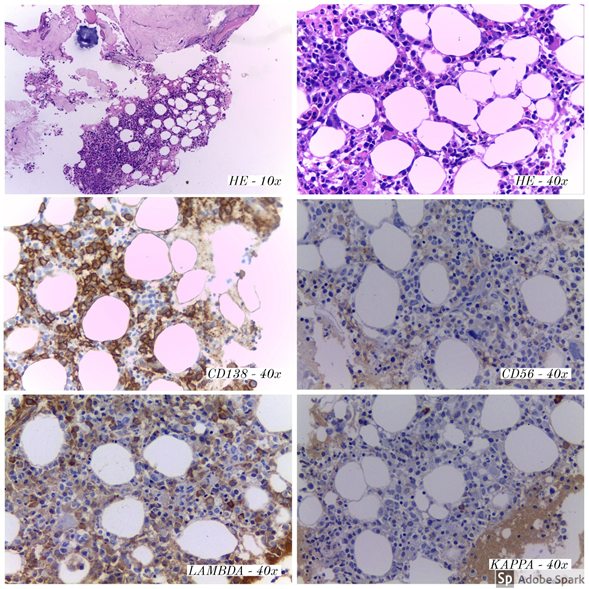
Figure 2 Bone marrow, trephine biopsy. Plasma cell aggregates (CD138 positive), with Lambda light-chain restriction and weak positivity for CD56.
Taking into account the previous approach and applying it to the clinical picture of our patient, in the medical history there is evidence of weight loss, fatigue, renal failure, hepatomegaly, without autonomic symptoms, which generates initial suspicion of POEMS syndrome. Electrodiagnostic studies revealed a demyelinating neuropathy the M protein analysis revealed an elevation of IgG/lambda, lambda being typically found in this syndrome. These collective data correspond to the two mandatory criteria for the diagnosis of POEMS syndrome, however, major criteria for diagnosis and only one minor criterion (hepatomegaly) were met, for which reason aspirate and bone marrow biopsy are performed to consider an alternative etiology.
Complementary studies: In the study of paraproteinemic neuropathy with systemic involvement, bone marrow aspiration and biopsy with myelogram and flow cytometry are essential, in order to determine the percentage of infiltration and the clonality of plasma cells.
In this patient, a bone marrow study was carried out that showed infiltration by 15.7% of plasma cells with pathological IgG/Lambda immunophenotype, which, associated with the presence of renal failure, hypercalcemia, and anemia, allowed to establish the diagnosis of Multiple Myeloma.
Given that during the evolution of the disease, he initially presented with abdominal pain and neurogenic bladder, over-aggregated amyloidosis was suspected and a colonoscopy was performed with a biopsy taken for Congo Red and Violet Crystal staining, which was negative for amyloid deposit.
Questions to consider:
What is the relationship between HIV infection and multiple myeloma?
There is a well-established relationship between HIV infection and plasma cell neoplasms, though the mechanism by which patients with HIV are at higher risk of developing them is not yet understood and a direct effect of the virus that favors the development of multiple myeloma has not been identified. Some hypotheses have been raised, such as antigenic stimulation of the virus that leads to B cell proliferation and immunoglobulin secretion without the help of T cells, immunodeficiency has also been raised as a facilitating factor.9
A meta-analysis that included 7 studies (n = 444,172) of patients with HIV looking for the incidences of different neoplasms reported a standardized incidence rate for multiple myeloma of 2.71 (95% CI 2.13 to 3.44).10 Another meta-analysis also revealed an increased risk of multiple myeloma in HIV/AIDS patients with a relative risk in the range of 1.9 to 6.5.14
The above suggests a relationship between the two entities, indicating that our patient's findings were not incidental.
With the above, it can be concluded that paraproteinemic neuropathies should be studied in cases of unknown etiology since they represent 10% of cases. However, other more common etiologies such as diabetic neuropathy should always be ruled out. The approach to paraproteinemic neuropathies should be systematic based on clinical history, electrodiagnostic studies and elevated M protein type, which in most cases will lead to an accurate diagnosis. However, there are cases such as that of our patient who, despite indications that led to a specific entity, complementary studies led to a different diagnosis, indicating the need for an individualized approach with high diagnostic suspicion and complete studies of these patients.
None.
The author declares that there are no conflicts of interest.

©2025 Cartagena, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.