Journal of
eISSN: 2376-0060


Short Communication Volume 12 Issue 1
School of Medicine, Universidad de Buenos Aires (UBA), Argentina
Correspondence: Adrian Hunis, School of Medicine, Universidad de Buenos Aires (UBA), Argentina
Received: May 05, 2025 | Published: May 29, 2025
Citation: Hunis M, Hunis A. Current status of molecular markers in diagnosis, treatment, and prognosis of non-small cell lung cancer (NSCLC). J Lung Pulm Respir Res. 2025;12(1):13-16. DOI: 10.15406/jlprr.2025.12.00325
Non-small cell lung cancer (NSCLC) accounts for the vast majority of lung cancer cases and remains a leading cause of cancer mortality worldwide. Advances in molecular biology have revolutionized the diagnosis, treatment, and prognosis of NSCLC through the identification of key molecular markers. These markers, including driver mutations (such as EGFR, ALK, ROS1, BRAF, KRAS, MET, RET, and HER2) and immune-related biomarkers (like PD-L1 and tumor mutational burden), enable personalized therapeutic strategies and improved patient outcomes. Comprehensive molecular profiling using techniques such as next-generation sequencing, immunohistochemistry, and liquid biopsy is now standard in clinical practice. Targeted therapies and immunotherapies have significantly increased response rates and survival, particularly in patients with actionable mutations or high PD-L1 expression. However, most advanced NSCLC cases remain incurable due to the development of resistance mechanisms, although curative outcomes are possible in early-stage disease with surgery and adjuvant targeted therapy. Ongoing research focuses on overcoming resistance, expanding biomarker panels, and integrating novel therapeutic approaches. The future of NSCLC management lies in precision medicine, multidisciplinary care, and continued innovation in molecular diagnostics and targeted treatment.
Non-small cell lung cancer (NSCLC) represents about 85% of all lung cancer cases, with the remainder being small cell lung cancer (SCLC). NSCLC is further subdivided into adenocarcinoma (the most common, especially in non-smokers), squamous cell carcinoma, and large cell carcinoma. Lung cancer is the leading cause of cancer death worldwide, accounting for nearly 1 in 5 cancer deaths.
|
Parameter |
Value (2020) |
|
Global new cases |
2206771 |
|
Global deaths |
1796144 |
|
NSCLC % of lung cancers |
~85% |
|
Median age at diagnosis |
70 years |
|
5-year survival |
25% (all stages) |
|
% diagnosed at stage IV |
>50% |
Table 1 Epidemiology of NSCLC
Types and classification
Molecular markers (MM) are genetic, epigenetic, or protein alterations that provide information about tumor biology, prognosis, and potential therapeutic targets. They are classified as:
EGFR, ALK, ROS1, BRAF, KRAS, MET, RET, NTRK, HER2
PD-L1, Tumor Mutational Burden (TMB), MSI9
TP53, STK11, KEAP1 (Tables 2&3) (Figure 2).
|
Marker |
Type |
Frequency (%) |
Associated Subtype |
Actionable? |
Targeted Therapy Available |
|
EGFR |
Driver |
10-15 (Caucasian) 40 (Asian) |
Adenocarcinoma |
Yes |
Yes |
|
ALK |
Driver |
3-7 |
Adenocarcinoma |
Yes |
Yes |
|
KRAS |
Driver |
25-30 |
Adenocarcinoma |
Yes (G12C) |
Yes |
|
ROS1 |
Driver |
1-2 |
Adenocarcinoma |
Yes |
Yes |
|
BRAF |
Driver |
1-4 |
Adenocarcinoma |
Yes (V600E) |
Yes |
|
MET |
Driver |
3-4 |
Adenocarcinoma |
Yes |
Yes |
|
RET |
Driver |
1-2 |
Adenocarcinoma |
Yes |
Yes |
|
HER2 |
Driver |
1-3 |
Adenocarcinoma |
Yes |
Yes |
|
NTRK |
Driver |
<1 |
Adenocarcinoma |
Yes |
Yes |
|
PD-L1 |
Immune |
25-60 |
All |
Yes |
Yes |
|
TMB |
Immune |
Variable |
All |
Yes |
Yes |
|
TP53 |
Prognostic |
50 |
All |
No |
No |
|
STK11 |
Prognostic |
10-20 |
Adenocarcinoma |
No |
No |
Table 2 Major Molecular Markers in NSCLC
|
Feature |
"Hot" Tumors |
"Cold" Tumors |
|
TMB |
High |
Low |
|
PD-L1 |
High |
Low |
|
Immune Cells |
Abundant (CD8+ T cells) |
Sparse |
|
Common Mutations |
KRAS TP53 smoking-related |
EGFR ALK never-smokers |
|
Immunotherapy |
High response |
Low response |
Table 3 Molecularly “Hot” vs. “Cold” Tumors in NSCLC
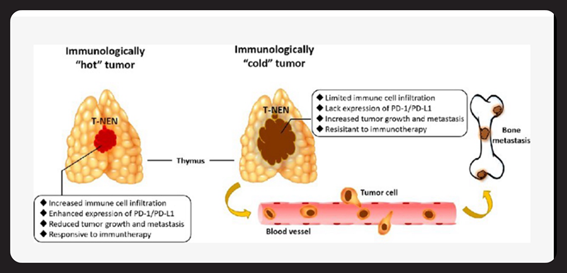
Figure 2 Hot vs. Cold Tumor Microenvironment (Schematic Illustration).
Diagram showing immune cell infiltration and PD-L1 expression in hot vs. cold tumors. (Frontiers in Oncology)
Tumor “Hot” and “Cold” Status
Diagnostic techniques
|
Method |
Markers |
Advantages |
Limitations |
|
PCR |
EGFR KRAS BRAF |
Fast sensitive |
Limited to known mutations |
|
FISH |
ALK ROS1 RET |
Detects rearrangements |
Labor-intensive expensive |
|
IHC |
ALK ROS1 PD-L1 |
Widely available inexpensive |
Subjective interpretation |
|
NGS |
Multiple genes/fusions |
Comprehensive detects rare events |
Cost technical expertise needed |
|
Liquid Biopsy |
ctDNA (EGFR ALK etc.) |
Non-invasive real-time monitoring |
Lower sensitivity false negatives |
Table 4 Methods for Molecular Marker Detection
Prognostic value
|
Marker |
Prognostic Value |
Predictive Value |
Therapy Impact |
|
EGFR |
Favorable (with TKI) |
Predicts TKI response |
High |
|
ALK |
Favorable (with TKI) |
Predicts TKI response |
High |
|
KRAS |
Unfavorable |
Predicts G12C inhibitor |
Moderate |
|
PD-L1 |
Variable |
Predicts immunotherapy |
Moderate-High |
|
STK11 |
Unfavorable |
Poor immunotherapy response |
Low |
Table 5 Prognostic and Predictive Value of Key Markers
Targeted therapies and immunotherapy
The identification of actionable mutations has revolutionized NSCLC therapy. Patients with specific mutations receive targeted therapies, while those with high PD-L1 or TMB may benefit from immune checkpoint inhibitors (Table 6) (Figure 3).
|
Marker |
Drug(s) |
Response Rate (%) |
Median PFS (months) |
Median os (months) |
|
EGFR |
Osimertinib, Erlotinib |
60-80 |
10-19 |
30-38 |
|
ALK |
Alectinib, Crizotinib |
60-80 |
10-34 |
34-57 |
|
ROS1 |
Crizotinib, Entrectinib |
70-80 |
15-20 |
30-40 |
|
BRAF V600E |
Dabrafenib+Tra metinib |
60-70 |
9-10 |
18-24 |
|
MET |
Capmatinib, Tepotinib |
40-50 |
6-12 |
17-21 |
|
RET |
Selpercatinib, Pralsetinib |
60-70 |
17-24 |
30-40 |
|
KRAS G12C |
Sotorasib, Adagrasib |
30-45 |
6-8 |
12-15 |
|
PD-L1 >50% |
Pembrolizumab (mono) |
40-45 |
7-10 |
20-26 |
Table 6 Targeted Therapies and Clinical Outcomes in NSCLC
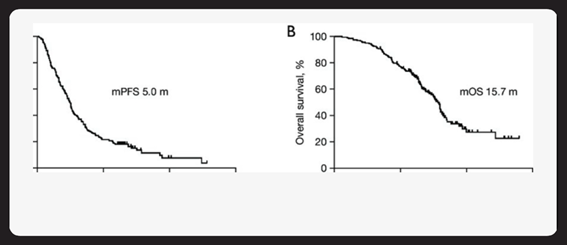
Figure 3 Progression-Free Survival by Molecular Subtype (Kaplan-Meier curves).
Kaplan-Meier curves of PFS (A) and OS (B) of patients with advanced NSCLC who received ICl-based treatment beyond progression with prior immunotherapy. mPFS, median progression-free survival; mOS, median overall survival; NSCLC, non-small cell lung cancer; ICI, immune checkpoint inhibitor.
In advanced NSCLC, targeted therapies and immunotherapies have greatly improved outcomes, but true cures are rare due to the development of resistance. In early-stage disease, adjuvant targeted therapy (e.g., osimertinib for EGFR-mutant NSCLC) has increased the chance of cure, as shown in the ADAURA trial.2 However, long-term follow-up is needed to confirm durable cures.3-7
|
Stage |
Curability |
Role of Molecular Markers |
|
I-II |
High |
Adjuvant targeted therapy improves DFS |
|
III |
Moderate |
Multimodal therapy + immunotherapy |
|
IV |
Low |
Rare durable responses, not curable |
Table 7 Curability by Stage and Molecular Profile
Molecular markers have transformed the landscape of NSCLC, enabling precision medicine that improves survival and quality of life. While most advanced cases remain incurable, ongoing research into novel targets, resistance mechanisms, and combination strategies offers hope for further progress. Early detection, comprehensive molecular profiling, and access to innovative therapies are key to improving outcomes.
None.
Authors declare no conflict of interest.

©2025 Hunis, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.