Journal of
eISSN: 2374-6947


Mini Review Volume 7 Issue 1
1Department of Biochemistry, Madonna University Okija, Nigeria
2Department of Biochemistry, University of Ibadan, Nigeria
Correspondence: Nwawuba Stanley Udogadi, Department of Biochemistry, Faculty of Natural Science, Madonna University, Okija, Nigeria, Tel +234-8065699068
Received: February 21, 2020 | Published: March 20, 2020
Citation: Udogadi NS, Abdullahi MK. Interplay between nutrigenomics and diabetes: a mini review.J Diabetes Metab Disord Control. 2020;7(1):9-12. DOI: 10.15406/jdmdc.2020.07.00194
Diabetes Mellitus, regarded as a silent killer, has been demonstrated to be a prime global health concern with a projected rise in prevalence from 171 million in 2010 to 366 million in 2030. Correspondingly, dietary management has shown to be a cornerstone modality in the attainment of good glycemic control in diabetes, and Nutrition/Diet remains a key player in diabetes prevention and management. In this light, of course, evidences from prospective observational studies, clinical trials and experimental findings have reported ameliorating ability of a number foods and nutrient on diabetes. However, a major concern is the genetic variability of individuals and their responses to these functional foods. Therefore, it has become necessary to understand how nutrients act at the molecular level which in turn involves a cascade of nutrient-related interactions at the gene, protein and metabolic levels. Overall, a Nutrigenomic approach provides; a snapshot showing genes that are switched on/off (the genetic potential) at any given moment, and the method to determine the influence of nutrients on gene/protein expression. Nutrigenomic has opened a new future to screen the genetic background, to monitor the transcriptome, proteome and metabolome and to ultimately develop dietary strategies which are targeted to supply the optimum nutrition and therapeutics for single individuals. Therefore, it is vital to support researches in the field of nutrigenomics in response to the prevalence of diabetes mellitus. Hence, the present review provides an understandings vis-à-vis the role of nutrient-gene interactions in diabetes, treatment, prevention and pathogenesis.
Keywords: nutrigenomics, diabetes, nutrigenomics and diabetes, nutritional therapy, pancreatic
DM, diabetes mellitus; EGCG, epigallocatechin gallate; T1DM, type 1 DM; T2DM, type 2 DM; NCD, non-communicable diseases; HGP, human genome project; PDX1, pancreatic and duodenal homeobox 1; GLUT2, glucose transporter 2; SUR1, sulfonylurea receptor-1
Diabetes mellitus (DM) is a metabolic disorder resulting mainly from either the inability of the pancreas to secrete a hormone (insulin) that is responsible for mopping the level of blood sugar, or situation arising from the constellation of insulin insensitivity, resistant and excessive secretion of a hormone called glucagon.1 As previously reported, DM remains a world health challenge with an estimated increase in incidence ranging from 171 million in 2010 to 366 million in 2030.2 Both the number of cases and its prevalence has been indicated to be on a rapid increase over the years, and as a consequence, DM is considered a silent killer.2,3 In an attempt to reduce the menace of diabetes mellitus, several approached has been tested and adopted for the prevention and treatment, including nutritional therapy. However, a key challenge that demands attention is genetic variability. Therefore, an approach which recognizes the interplay between nutritional therapy and genetic factor has become very necessary. This necessity brings nutrigenomics to the forefront. Hence, nutrigenomics offers personalized nutritional advice, develops specialized diet/nutrient for a populations or for individuals, and in general, it is regarded as personalized nutrition for the treatment and prevention of several disease conditions including DM.3 A Nutrigenomic approach finds huge application to the questions of human health and disease, and it is key to understanding the intricacies of interplay between basic metabolic activities and external determinant in disease processes.4 It has been demonstrated that, nutrigenomics is critical for the advancement of more targeted strategies in the treatment and prevention of diseases.4
Therefore, nutrigenomics research is presently the mainstay owing to the fact that food-derived bioactive components considerably alters genomic, proteomic and metabolic changes.5 Nutrigenomics provides an understanding as to how bioactive compounds of a particular diet influences the expression of genes, leading to either activation or suppression.6,7 Following the understanding on the interplay between nutrition, genes and diseases provided by nutrigenomics, quite a number evidences exists to this effect. Mediterranean Diet, has been showed to reduce cardiovascular risk factors and stroke prevalence in people with polymorphisms strongly associated with type 2 DM,8 flavanol, epigallocatechin gallate (EGCG) has been demonstrated to guard insulin secretory cells from pro-inflammatory cytokine-induced toxicity via modulation of β cell CLL/lymphoma 2 (BCL-2) expression,9,5 and Biotin food sources including Spinach, egg, sweet potato and almonds, enhances insulin secretion and islet function via the gene interaction by signaling an increase in the following gene FOXA2 (Forkhead Box A2), HNF-4α a nuclear transcription factor, and Calcium Channel Neuroendocrine/Brain-Type, Alpha-1 Subunit (CACNA1D).10 Therefore, it is vital to support researches in the field of nutrigenomics in response to the prevalence of diabetes mellitus. Hence, the present research provides an understandings vis-à-vis the relationship between nutrition and gene interactions in the treatment, prevention and pathogenesis of diabetes.
Diabetes
Diabetes Mellitus (DM) regarded as a serious chronic disease, and its vastly categorized into two (2) etiopathogenetic: Type 1 DM (T1DM) and type 2 DM (T2DM), respectively).1,8 T1DM also considered as insulin-dependent diabetes11 or juvenile-onset diabetes, it’s a consequence of a pancreatic β cells dysfunction, and as a result, affected persons depends on exogenous insulin.8 One of the key player to development of T1DM, its genetic predisposition via environmental influences in the form of dietary components.12 On the other hand, T2DM is a consequence of situations arising from the constellation of insulin insensitivity, resistant and excessive secretion of a hormone called glucagon.1 Its etiology could be as a result of complex combinations of genes, environmental factor (diet) and the interplay between these genes and environmental factors.8 DM regarded as a serious public health challenge, remains among the top four priorities of non-communicable diseases (NCD) targeted for action by world leaders.1 Based on existing report, it has been demonstrated that the incidence of diabetes has rapidly been on the rise over the years and as such, it is described to be a silent killer disease affecting large population of people around the world.12 The rise in worldwide prevalence is reported to grow from 171 million in 2010,13 to 382 million in 2013,14 and it is expected to rise to 592 million by 2035.1,14 Clearly, DM poses as huge global health challenge that has assumed pandemic proportion affecting the developing countries more than the developed countries.1,12,13 Owing to this in part, the important role of diet/nutrient in diabetes management and prevention was recognized by the medical society particularly the American Diabetes Association and new recommendations were then focused on individualized eating plans targeted at reaching specific biomarkers.15
Nutrigenomic
Environmental factor including diet/nutrition is recognized to be a key contributor to human health and several disease conditions.16 As such, nutritional research has continued to elucidate and provide an understanding of how to maintain a good health, and effectively ameliorate several disease conditions using dietary approach. The completion of the Human Genome Project (HGP) led to advancement in nutritional approach, and the opening of frontier on targeted nutritional therapy. According to Sales et al.,6 the conclusion of HGP led to new insights on the role of diet/nutrition on human health, and as a result the following postulations were made;6
Nutrigenomics, sometimes called nutritional genomics is an emerging area of research, and in recent years, the field has received enormous attention.4 It focuses on the investigation on the area of nutrition that adopts molecular approaches to elucidate responses from certain diet exposed to either individual or population groups.17,18 A number of definitions on nutrigenomics exist, and the all tends towards same meaning; influence of nutrition/nutrient on an individual genome hence, nutrigenomics. Accordingly, Barnett & Ferguson3 nutrigenomics is considered as the interactions between diet/nutrition and an individual’s genome, and the downstream effects on his or her phenotype.3 Nutrigenomics offers personalized nutritional advice, develops specialized diet/nutrient for a populations or for individuals, and in general, it is regarded as personalized nutrition for the treatment and prevention of several disease conditions including DM.3 Nutrigenomics is critically involved with developing scientific framework to enhance a better understanding on genetic contributions to such preferences, requirements, and responses to diet/nutrition, and also advance future methods for consumers to assess their health and nutritional status.19,20 Correspondingly, it recognizes that a particular dietary choice for one person may be unsuitable for the other,3 and similar to its analogous, pharmacogenomics, nutrigenomics has been demonstrated to possess the ability of identifying genetic predictors that responds to diets in relation to disease conditions and its applicability in the milieu of personalized nutrition have popular appeal.4
Interplay between of nutrient/gene interactions (nutrigenomics) vis-à-vis diabetes mellitus
Several studies have shown and established the fact that diet/nutrition plays a prominent role in the etiology and prevalence of disease conditions including diabetes through an interaction of multiple genes in response to the diet.2,8 Therefore, the identification and analysis of the interaction between diet/nutrient and genes (nutrigenomics) are important steps toward the understanding of DM etiopathogenesis, prevention and management.8,3 In establishing the interplay between diet and gene interactions, nutrigenomics greatly relies on high-throughput technologies and methodologies in genomics, transcriptomics, proteomics and metabolomics for the analysis of targeted genes and metabolites that responds to the diet/nutrient, and also the analysis of bioactive compounds present in foods as well as how they affects the human metabolism, nutritional homeostasis and molecular events involved in nutrition-related diseases, such as diabetes.8 In molecular nutrition perspective, nutrients could be regarded as signaling molecule capable of either transmitting or translating dietary signals into changes in the gene expression as well as synthesis of proteins and metabolites via appropriate cellular sensing mechanisms.5 Generally, diet/nutrients has the ability to affect gene expression via the following mechanism as stated by Genoveva et al.,8 : (i) directly (ii) through their metabolites and (iii) through signal transduction molecules. This is depicted in figure 1, as the figure shows, food/diet/nutrient influences gene expression leading to prevention and treatment of diabetes (glucose hemostasis). The understanding of the interplay between diet/nutrient and human genome has helped establish precision nutritional therapeutic approaches to the study of health and disease.21 Clearly, the potentials provided by evidence-based personalized or precision nutritional therapeutic approaches towards the maintenance of good health as well as the amelioration of several disease states including diabetes, continues to be an important healthcare goal.3 Evidence has shown that several dietary-bioactive compounds possesses the ability to regulate gene expression directly or indirectly through induction of metabolites or signaling molecules that influence complex metabolic pathways involved in the development of diabetes.5
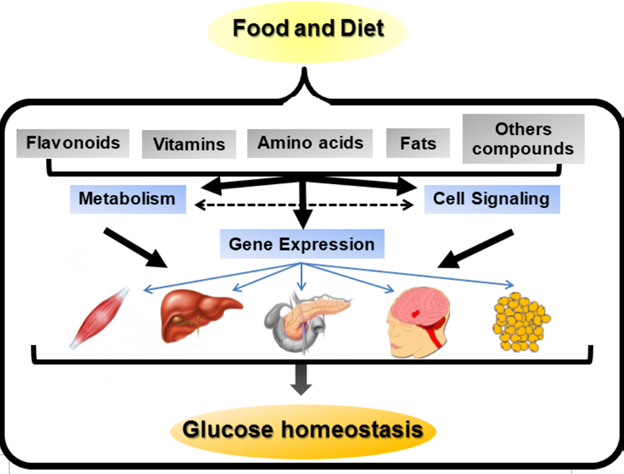
Figure 1 Diet-Gene expression mechanisms.8
In addition, quite a number of studies using humans, animals, and cell cultures model, have revealed the interplay between diet/nutrient and gene expression involved in diabetes. Naringin, present in large amount in citrus fruits, and some vegetables, has been demonstrated to reduce insulin resistance, improve β cell function, and acts as potent a hypoglycemic candidate via gene interaction by improving the peroxisome proliferator-activated receptors (PPPAR-γ),22 Biotin food sources including spinach, egg, sweet potato and almonds, enhances insulin secretion and islet function via the gene interaction by signaling an increase in the following gene FOXA2 (Forkhead Box A2), HNF-4α a nuclear transcription factor, and Calcium Channel Neuroendocrine/Brain-Type, Alpha-1 Subunit (CACNA1D),10 Taurine from animal foods, such as meat, fish and dairy as the main sources, increases Insulin secretion and insulin synthesis via gene interaction by signaling an increase in the following gene PDX1 (pancreatic and duodenal homeobox 1), Glucose transporter 2 (GLUT2), and sulfonylurea receptor-1 (SUR1) encoded by the ABCC8 gene,23 Dietary amino acids modulates the insulin resistant and type 2 diabetes mellitus by influencing gene and protein expression in pancreatic β- cell of islets of langerhans,24 Vitamin D promotes insulin secretion and cell protection by attenuating the expression and activity levels of IL-1, IL-6 and TNF-α in T2DM,5 naturally occurring bioactive chemicals including flavonoids, carotenoids, coumarins, and phytosterols; and zoochemicals such as eicosapentaenoic acid and docosahexaenoic acid) regulate gene expression in diverse ways,21 and Sorghum improves insulin sensitivity via (PPAR-γ) from adipose tissue, down-regulates of TNF-α, up-regulates adiponectin, and improves lipid profile status.25,2
The knowledge of how food interferes with the genetic code and how the organism responds to these interferences is what nutrigenomic brings. Therefore, it is imperative to improve research in the field of nutrigenomics because these discoveries will deliver a framework for the development of genotype-dependent food health promotion strategies/evidence-based personalized or precision medicine through dietary intervention, and the design of dietetic approaches for the prevention and management of diabetes mellitus.
None.
The authors declared that there are no conflicts of interest.
None.

©2020 Udogadi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.