Journal of
eISSN: 2373-4345


Research Article Volume 12 Issue 2
Department of Dentistry, School of Dentistry, Taubaté University (UNITAU), Brazil
Correspondence: Dr. Tribst JPM, School of Dentistry, Taubaté University (UNITAU), Operários street 09, Taubaté, SP, Brazil
Received: March 26, 2021 | Published: May 14, 2021
Citation: Rangel JHR, Zanatta RF, Albuquerque AL, et al. The effect of bleaching gel application on the physical properties of different CAD/CAM restorative materials. J Dent Health Oral Disord Ther. 2021;12(2):41-44. DOI: 10.15406/jdhodt.2021.12.00547
Objective: To evaluate the surface roughness and microhardness of CAD/CAM lithium disilicate, feldspathic ceramic, polymer infiltrated ceramic and nanohybrid composite before and after the application of bleaching gel.
Material and methods: Disc shaped specimens were polished and divided into quadrants for roughness (Ra) and microhardness (Vickers, 19.61 N, 20 s) measurements. The 35% hydrogen peroxide was applied simulating three in-office bleaching sessions.
Results: Paired t-test (α=0.05) showed that bleaching gel application did not affect the surface roughness of the tested material. The microhardness of polymeric materials (polymer infiltrated ceramic and nanohybrid composite) presented statistical significant increase (2 % increase, which may be clinically insignificant).
Conclusion: Microhardness of polymeric CAD/CAM materials may be affected by bleaching gel application.
Keywords: bleaching, dental ceramics, microhardness, surface roughness
Tooth whitening has been a procedure increasingly sought by patients who want to whiten their teeth and, given the satisfactory aesthetic results and conservative technique, it has been increasingly recommended by dentists.1 Like tooth hard tissues, restorative materials, both direct and indirect, may present extrinsic color alteration due to the incorporation of pigments from diet such as, wine, coffee, tea, smoke and others.2-4 The composition of the restorative material influences the intensity of the alteration.3-5 Therefore, bleaching recently has being seen as an option to remove these stains from these materials,3,4 and their replacement, due to discoloration, can be postponed or even may no longer be necessary.
The bleaching techniques vary according to the bleaching agent’s type, concentration, and technique which is classified as at-home, in-office or a combination between them. Regardless of the technique, the active principle of tooth whitening is hydrogen peroxide which degrades into free radicals oxidizing and reducing stains until partial or total elimination by diffusion.6,7 Both the technique and the peroxide type (hydrogen or carbamide) are effective to promote bleaching.8
Materials for computer aided manufacturing and computer aided machining (CAD/CAM) are supposed to be void-free materials,9 perhaps impairing bleaching agent action/diffusion. Feldspathic ceramic (VITABLOCS Mark II for Cerec, Vita Zahnfabrik, Germany) was reported not to suffer alterations in color or surface characteristics after bleaching protocol (two 20-min applications of 40% hydrogen peroxide gel in each session, in a total of 2 sessions).5 But the composition of ceramics influences the materials properties, such as microhardness, fracture toughness, slow crack growth,10 resistance to chemical degradation,11 adhesive potential,12,13 and reaction to staining and bleaching protocols.5
Even if the intention is not to bleach the restorative material, the bleaching agent may have contact with the restorative material surface during tooth bleaching, leading to alteration on surface characteristics of the materials. Literature has no consensus regarding this topic. Bleaching is reported not to affect microhardness14 and roughness,15 as to decrease hardness in feldspathic ceramics,16 lithium disilicate and zirconia,17 and increase in roughness in leucite glass ceramic.18 Time of exposition to bleaching agent,19 bleaching protocol and type of restorative material5 may influence the effects of bleaching in materials surface.
The aim of this study was to evaluate the surface roughness and microhardness of four different CAD/CAM materials before and after the application of 35% hydrogen peroxide. The null hypothesis is that the tested materials do not suffer alteration in surface roughness and microhardness after the application of bleaching agent.
Study design
Disc-shaped samples were manufactured from each tested material (n=5); lithium disilicate (IPS e.max CAD Ivoclar Vivadent, Schaan, Lieschtenstein); feldspathic ceramic (Vita Blocks Mark II Vita Zahnfabrik, Bad Säckingen, Germany); polymer infiltrated ceramic (VITA Enamic Vita Zahnfabrik, Bad Säckingen, Germany); and nanohybrid composite (Brava Block FGM, Joinville, Brazil). They were mirror polished and received the application of bleaching agent (35% hydrogen peroxide, 35% Whiteness HP Maxx, FGM, Joinville, Brazil) during 15 min in three consecutive times. This protocol represented one session, according to manufacturer instructions, and a total of three sessions were performed. Vickers microhardness and surface roughness were evaluated before and after the application of bleaching agent.
For samples’ fabrication, CAD/CAM blocks were hand-rounded into 11 mm diameter cylinders in polishing machine (Aropol E, Arotec, Cotia, Brazil), with #600-grit sandpaper. Thereafter, cylinders were sectioned into 1 mm discs in a precision saw machine (Isomet 1000, Buehler, Lake Buff, USA). Lithium disilicate discs were crystalized in specific furnace (Programat EP 5000, Ivoclar Vivadent, Schaan, Lieschtenstein, crystalizing cycle: 820ºC/10 s, 840°C/7 min).
Disc-shaped specimens were embedded into chemically cured acrylic resin (JET, Classico, São Paulo, Brazil), with a 25-mm diameter, 10-mm high plastic matrix. Exposed surface of materials’ discs was polished with sandpaper for 1 min with 400-, 600-, and 1200-grit, respectively. Embedded and polished samples were subjected to ultrasonic bath (Cristófoli, Campo Mourão, Brasil) of distilled water for 3 min. A reference mark was performed with a permanent marker for identification of specific sites in the disk where the tests were performed (Figure 1).
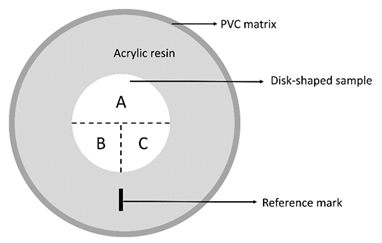
Figure 1 Representation of the disk-shaped sample, embedded into acrylic resin. The reference mark defined the sites for evaluation of surface roughness (A) and microhardness before (B) and after (C) bleaching gel application.
Surface Roughness and Microhardness measurements
Initial surface roughness (Ra) was measured by a contact profilometer (Surftest SJ 310, Mitutoyo, Tokyo, Japan). Three parallel measurements (ʎc 0.25 mm) were performed on the pre-determined sites (Figure 1). The mean value of the three obtained surface roughness values (Ra) was considered the sample surface roughness before the application of bleaching agent.
Initial microhardness was measured by a microhardness tester (Shimadzu MicroHardness Tester HMV G20, Shimadzu Corporation, Kyoto, Japan). Three Vickers indentations were performed on the pre-determined site (figure 1) during 20 s, with 19.61 N load. The mean value of the three obtained microhardness values (VHN) was considered the sample microhardness before application of bleaching agent.
Bleaching protocol
Hydrogen peroxide 35% (Whiteness HP Maxx 35 %, FGM, Joinville, Brazil) was mixed according to manufacturer instruction and applied to the samples surface simulating three in-office bleaching sessions. Each session comprised three applications of bleaching agent to the material surface during 15 min and alternated by abundant washing with distilled water. During the time between each session and measurements, samples were stored into distilled water to avoid dehydration.
The surface roughness and microhardness were measured after the application of bleaching agent in the specific sites (Figure 1), as mentioned before. Values obtained were subjected to paired t-test (α=0.05), comparing each material before and after application of bleaching gel.
Scanning electron microscopy
Representative samples of each material before and after bleaching agent application were prepared for the observation under scanning electron microscopy (SEM). Samples were gold-sputter coated (SC7620 Mini Sputter Coater, EMITECH, Laughton, Montigny-le-Bretonneux, France) and the surface pattern was observed in SEM (Inspect S50, FEI, Hillsboro, USA) under 5.000 × magnification.
The application of whitening gel did not affect roughness for any of the tested material. However, the surface microhardness was enhanced for the polymer-infiltrated ceramic and the nanohybrid composite (Table 1). Representative images showed no alteration in surface pattern for any of the tested materials after application of bleaching agent (Figure 2).
Tested materials |
Surface roughness |
p-value* |
Microhardness |
p-value* |
||
Before |
After |
Before |
After |
|||
Lithium-disilicate ceramic |
0.213 ±0.06 |
0.234 ±0.15 |
0.808 |
796.1 ±42.1 |
778.4 ±27.3 |
0.106 |
Feldspathic ceramic |
0.170 ±0.06 |
0.279 ±0.22 |
0.380 |
722.5 ±60.5 |
749.9 ±48.5 |
0.093 |
Polymer-infiltrated ceramic |
0.161 ±0.04 |
0.165 ±0.02 |
0.682 |
628.7 ±70.3 |
697,4 ±58.3 |
0.001 |
Nanohybrid composite |
0.141 ±0.02 |
0.167 ±0.06 |
0.422 |
79.53 ±2.33 |
80.84 ±2.28 |
0.031 |
Table 1 Surface roughness (µm) and microhardness values (VH) of each tested material before and after the application of whitening gel solution, and respective statistical significance
*α=0.05
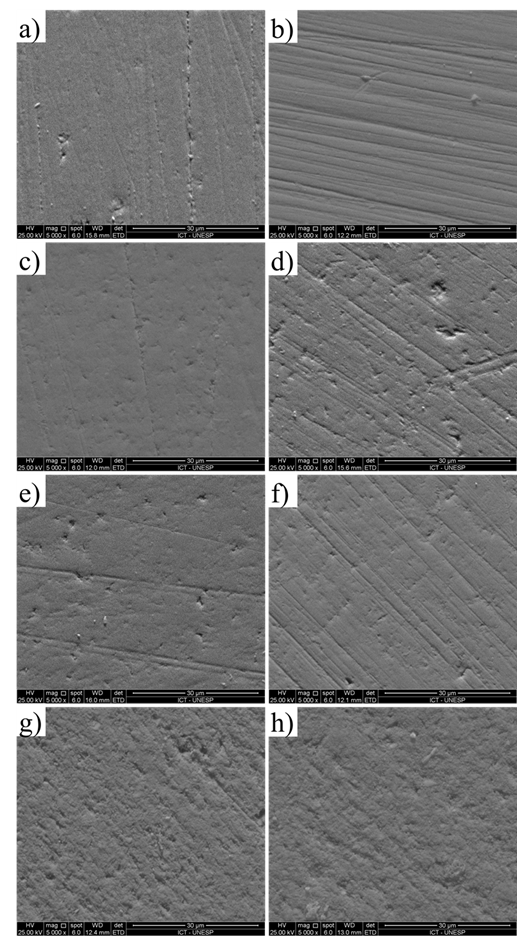
Figure 2 SEM representative images (5.000 × magnification) of the materials’ surface: a) Lithium-disilicate before bleaching agent application; b) Lithium-disilicate after bleaching agent application; c) Feldspathic ceramic before bleaching agent application; d) Feldspathic ceramic after bleaching agent application; e) Polymer-infiltrated ceramic before bleaching agent application; f) Polymer-infiltrated ceramic after bleaching agent application; g) Nanohybrid composite before bleaching agent application; and h) Nanohybrid composite after bleaching agent application.
The resultant effect of bleaching gel application on the tested materials was the enhancement of microhardness of polymer-infiltrated ceramic and nanohybrid composite, thus rejecting the null hypothesis (Table 1). The literature lacks consensus regarding surface alterations for restorative materials after bleaching regimens, both with carbamide and hydrogen peroxides.17,20-23
The increase in microhardness of direct composites after the application of bleaching agents is seldom reported in literature.20 Mainly, it is attributed to the dissociation of the polymeric chain by the free radicals released from the hydrogen peroxide, which brakes the double bonds from the cross linked chains, fomenting physical and chemical degradation. The oxidative free radicals from the bleaching gels may disintegrate the interface between inorganic matrix and polymeric matrix.23 This results in the decomposition of polymeric matrix and monomers release from composite,24 and the high amount of inorganic matrix on the materials surface could lead to an enhance of surface microhardness.
As far as we are concerned, this is the first study testing a nanohybrid composite for CAD/CAM and according to the respective manufacturer, it is composed by methacrylic monomer with glass ceramic filling (58% in volume). Although its composition resembles a direct composite, it presents far more conversion degree of the monomers, which is claimed to give it more mechanical and physical resistance. The microhardness increase after bleaching, although statistically significant, was around 2% and the clinical relevance of this is questionable. Regarding the surface roughness, there was no difference, which might be explained by the lower and very superficial amount of organic matrix dissociated, not enough to affect the surface roughness. As demonstrated in figure 2, no significant alteration may be observed in samples before and after bleaching gel application. To avoid interference of the microhardness alteration after the bleaching, a simple polishing might be sufficient to remove this most superficial layer in this material.
The polymer infiltrated ceramic also contains polymeric molecules (UDMA and TEGDMA, according to manufacturer). Its amount of inorganic filler (feldspar enriched with zinc oxide, 75% in volume) is greater than the nanohybrid composite (58% in volume), also evidenced by the higher surface microhardness than nanohybrid composite (Table 1). Thus, both polymeric materials tested in the present study had the microhardness affected by the action of peroxides in the organic matrix.
Both tested ceramics did not have surface roughness and microhardness affected by bleaching gel application in the present study (Table 1), corroborating with reports in literature.5 Due to the organic matrix, resin composites are more prone to chemical degradation than ceramics.25 Ceramics are claimed to present chemical inertness. But they are reported to be degraded by organic acids.11 Also, after2 immersion in staining solutions, ceramics have suffered color alteration,26 which could be partially reversed by the application of bleaching protocols.3 Thus, the absence of alteration in the materials properties would be a good response.
Ceramics were also found to present surface16,18 and color alteration3,16 after contact with bleaching gels. The differences between studies may be attributed to the applied measurement method, and type and concentration of bleaching agent and bleaching protocol.5 Moreover, the effects of bleaching on materials characteristics are material-dependent and should be considered for each specific material to be exposed to bleaching agents.5,27
The present study evaluated four materials with different compositions, but there are much more materials available in the market. In addition, only one bleaching agent and its respective bleaching protocol were tested, thus the results obtained are restricting to these conditions. Besides that, hybrid ceramics show an unfavorable wear pattern over time,28 and a rough surface may behave differently under different chemical stresses compared to a maiden smooth surface (i.e. ceramics). The effects of the application of bleaching agents on restorative materials, regarding color alteration, surface and mechanical properties are not well understood, as the clinical impact of the alterations has been shown by in vitro studies.
Within the limitations of the present study, it is possible to conclude that the application of bleaching agent did not affect surface roughness of different indirect materials. However, the surface microhardness of polymer-infiltrated ceramic and nanohybrid composite was enhanced.
None.
None.
No potential conflict of interest relevant to this article was reported.

©2021 Rangel, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.