Journal of
eISSN: 2373-4345


Research Article Volume 16 Issue 2
Master and Specialist in Orthodontics, President of ABOL (Brazilian Lingual Orthodontics Association), Board of Orthodontics (ABOR – Brazilian Orthodontics Association), Private Clinic in Ribeirão Preto, Brazil
Correspondence: Henrique Bacci, M.Sc. Av. Braz Olaia Acosta, 727, Sl 504, Jd Califórnia, Ribeirão Preto, São Paulo, Brazil
Received: March 20, 2025 | Published: April 18, 2025
Citation: Bacci H. Effects of modifying the post-curing protocol on transparency and the fit capacity of directly printed expanders for pediatric patients. J Dent Health Oral Disord Ther. 2025;16(2):33-36. DOI: 10.15406/jdhodt.2025.16.00639
This study investigates the impact of post-curing protocol modifications on the transparency of directly printed expanders and evaluates the adaptive capacity of Shape Memory Aligners® for pediatric palatal expansion. Two encapsulated expanders were fabricated using Tera Harz TC-85 resin, based on digital models from a 6-year-old patient with skeletal maxillary atresia. One expander followed the recommended post-curing protocol, while the other underwent a 12-hour delay before post-curing. Transparency testing revealed significant opacity and yellowing in the delayed sample. Adaptation tests were conducted by applying a 2.4 mm transverse expansion and assessing fit under oral-simulated conditions. Results showed that the expanders maintained adaptation without attachments, suggesting that Tera Harz TC-85 aligners can accommodate clinically relevant expansions. The findings underscore the critical role of timely post-curing for optimal esthetics and support the clinical feasibility of using shape-memory aligners for progressive palatal expansion. Future clinical studies are recommended to explore different expansion magnitudes and the use of attachments to enhance retention.
Keywords: orthodontics, smart polymers, pediatric clear aligners, orthodontic appliance, 3D printers, dentistry, digital workflow
Palatal expansion is a widely used technique in Orthodontics for correcting transverse maxillary deficiencies, particularly during the active growth phase in children. This procedure aims to transversely enlarge the upper arch, promoting balanced occlusal development and harmonization of facial aesthetics. Traditionally, fixed expansion devices, such as the Hyrax appliance, have been employed for this purpose, utilizing a centrally positioned screw gradually activated to achieve mid-palatal suture separation.
Recently, Align Technology introduced a removable approach to palatal expansion, termed Incremental Palatal Expansion® (IPE), which relies on directly printed removable devices. This system is characterized by sequential use of removable expanders that provide successive increments of 0.25 mm per appliance, aiming at mid-palatal suture separation in young patients.
In the context of this emerging technology, the present study aims to evaluate the feasibility of applying encapsulated expanders produced with shape-memory properties (Shape Memory Aligners®) for palatal expansion in children. Initially, the experimental investigation will assess the effects of deliberate modifications in the post-curing protocol on the optical properties of these devices. Subsequently, the adaptive capacity of these appliance in response to progressive transverse increments will be evaluated, aiming to initiate a clinical protocol for palatal expansion using this technology.
For this study, a digital model of the upper dental arch was selected from a male patient, aged 6 years and 3 months, presenting deciduous dentition and skeletal maxillary atresia (Figure 1).
The virtual planning (setup) was performed using dentOne software (Diorco, Seoul, South Korea). An exclusive buccal movement of 1.2 mm was applied to each tooth, specifically targeting the deciduous molars and upper canines (Figure 2A&2B).
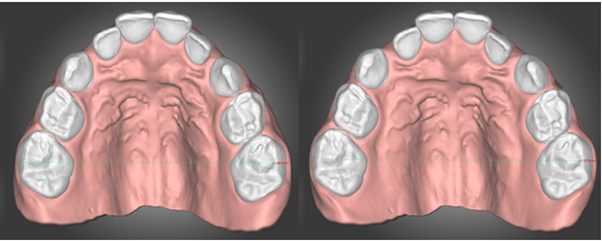
Figures 2A and B A presents Model 1 (patient's original), and B shows Model 2, in which a 1.2 mm buccal movement was applied to the upper deciduous canines and molars, resulting in a total expansion of 2.40 mm.
• Model 2: 2.40 mm expansion (intermolar distance = 47.0 mm)
Models 1 and 2 were exported and used for the configuration of two encapsulated expanders. The expanders were designed using DAD software (Direct Align Design, Graphy, Seoul, South Korea), based on Models 1 and 2, creating Expanders 1 and 2, respectively. The expander files in STL format were then exported (Figure 3) and transferred to slicing software for preparation of 3D printing.
After the slicing process, both expanders were printed using Tera Harz TC 85 resin. The expander from Model 1, after centrifugation, was intentionally left waiting for 12 hours before undergoing the post-curing sequence, including centrifugation, curing under nitrogen, vibration in water at 80°C, and boiling. The expander from Model 2 followed the same complete protocol recommended by the manufacturer, except it was performed immediately after the 3D printing.
Part 1 -transparency test
Visual differences between the two samples were observed. Expander 1, which underwent the post-curing treatment 12 hours after 3D printing, appeared opaque and yellowish, likely accompanied by changes in mechanical properties (not assessed in this study). Expander 2 was completely transparent, without signs of opacity (Figure 4).
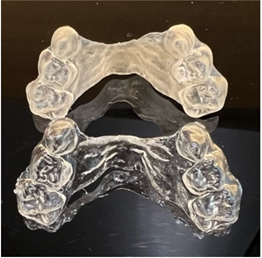
Figure 4 Above, Expander 1, for which the post-curing process was delayed by 12 hours. Transparency was severely impacted compared to Expander 2, where the process followed the manufacturer's recommendations. .
Part 2 -evaluation of appliance adaptation to transverse increments
Two approaches were conducted using the expanders produced in this study to evaluate appliance adaptation under different magnitudes of transverse expansion. In the first approach, Expander 1 (manufactured based on the patient's original dental arch) was tested for its adaptation to Model 5 (expanded by 2.4 mm). In the second approach, the adaptation of Expander 5 (manufactured based on the model already expanded by 2.4 mm) was assessed. It was fitted onto the patient's original, non-expanded arch. All adaptation evaluations were performed manually by an Orthodontist operator, with expanders immersed in water heated to 37°C, simulating oral conditions (Figure 5).
After this step, both sets were removed from the container and visually analyzed regarding their adaptation, in response to the applied transverse increment, as shown in Figures 6A-6C.
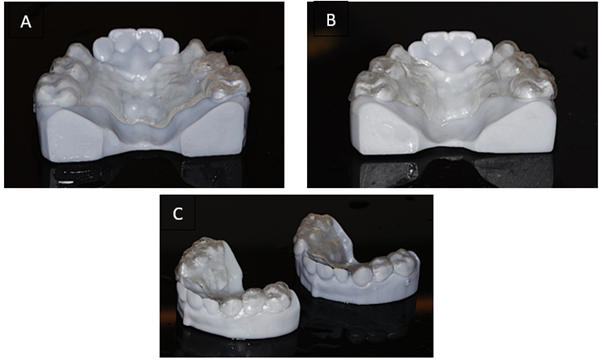
Figures 6A-C View of Expander 1 positioned on Model 2 in A; Expander 2 positioned on Model 1 in B. In C, both models with adapted expanders are shown from another perspective.
Figures 6A-C – View of Expander 1 positioned on Model 2 in A; Expander 2 positioned on Model 1 in B. In C, both models with adapted expanders are shown from another perspective.
The first part of this experimental study assessed the appearance of directly printed expanders, employing an intentional modification to the original recommended protocol. It is important to emphasize that, according to the literature,1-6 the entire production process of directly printed aligners should follow a continuous production sequence. This procedure aims to minimize surface exposure to oxygen, a factor known to compromise the optical stability of the devices. The marked color change observed in Expander No. 1, which remained on hold for 12 hours before undergoing the post-curing laboratory stage, confirms the importance of strictly adhering to the recommended protocol to maintain device transparency. It should also be noted that this study did not evaluate potential changes in mechanical properties resulting from the intentional alteration of the production protocol.
For removable expanders to exhibit adequate functional performance, effectively controlling dental and skeletal movements, it is essential that patients fully adhere to the recommended wearing regimen. Align Technology, for instance, recommends that Incremental Palatal Expanders (IPEs) be worn full-time, including during meals, and removed only for oral hygiene7 (brushing and flossing).
This recommendation aims to ensure maximum tracking accuracy and clinical effectiveness of the device. In the case of expanders produced by Align, it is possible to achieve 0.25 mm of expansion per stage in young patients, with replacements monitored by the Orthodontist.7 Daily changes allow for gradual and controlled separation of the mid-palatal suture, minimizing undesired displacements. Although this approach is still under investigation and current studies remain preliminary, the company has widely promoted this technology as a more comfortable alternative to conventional expanders. However, additional clinical studies are needed for definitive validation of the approach.
In the present study, directly printed expanders were fabricated using Tera Harz TC-85 resin, a polymer with shape-memory properties that allows customization according to the Orthodontic prescription. The flexibility of this material permits adjustments in thickness and structure extension based on individual biomechanical requirements. This experiment proposed the use of thermo-responsive shape-memory polymers, which recover their original form when exposed to a specific thermal stimulus.8
In the second phase of this investigation, encapsulated expanders were developed, with tests aimed at their clinical adaptation and the establishment of safe application parameters. The 0.3 mm increment per stage, previously analyzed in the literature, showed minimal variations in force intensity, suggesting the clinical safety of the protocol.9 Additionally, the directly-printed aligners exhibited significantly low forces throughout the entire range of tested displacement -a key aspect for reducing the risk of adverse effects in young patients with developing bone structures.
The Invisalign Palatal Expander (IPE), developed by Align, uses polyamide-12 (Nylon 12) as its base material. Its production process differs from that of the direct-print aligner made with liquid resin7. Instead of resin, it involves fused powder, typical of SLS (Selective Laser Sintering) printers. The manufacturer does not provide information regarding mechanical properties related to shape memory. From an aesthetic standpoint, the IPE material has a relevant limitation: the expander displays a whitish coloration.
On the other hand, expanders produced with Tera Harz TC-85 resin—provided the production protocol is followed- have shown excellent transparency. The shape memory properties of this material suggest that the limited staging of conventional materials can be overcome, opening the possibility of using a single shape-memory appliance instead of three successive conventional appliances.10 Moreover, it has been demonstrated that Tera Harz TC-85 exhibits highly effective shape memory at oral temperature, enabling greater tooth movement per stage.11,12
In this study, the fit of directly printed expanders- evaluated in an environment with temperature like the oral cavity- showed that expansions of up to 2.4 mm did not compromise the adaptive stability of the devices. Attachments were not used to enhance retention, although their inclusion may represent an additional strategy to improve clinical adaptation in real patient scenarios.
Expansions with different magnitudes from those used in this study may be considered in future clinical investigations. Considering that Align Technology recommends 0.25 mm increments per stage for its expanders (totaling 0.5 mm per step) and considering the approximate equivalence of one directly printed aligner to three thermoformed ones, it is proposed that increments of up to 0.75 mm per stage (totaling 1.5 mm) may be clinically feasible for direct-print expansor. This approach may also promote a more precise fit of the appliance, thereby providing greater clinical control over the expansion procedure.
As previously noted, the forces generated during activation with expanders produced using Tera Harz TC-85 do not reach levels that would pose a significant biological risk. Future clinical trials could help determine whether this activation protocol- or alternative protocols- as well as the replacement intervals for expanders made with this material, are clinically valid.
The post-curing protocol time recommended by the manufacturer must be strictly followed to prevent any changes in the appearance of appliances produced with Tera Harz TC-85.
Encapsulated expanders printed with this resin demonstrated that activations of 2.4 mm did not cause adaptation issues. Nonetheless, clinical studies could be conducted using different expansion magnitudes and the application of attachments in patients. Furthermore, large-scale validation, additional mechanical tests, and longitudinal clinical studies will be critical to confirm the long-term performance and safety of these devices.
None.
The author declares a potential conflict of interest that is remote and unrelated to the present study.

©2025 Bacci. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.