Journal of
eISSN: 2373-4345


Research Article Volume 14 Issue 1
1Professor of Dentistry at the University of São Francisco, Brazil
2Dental Students, São Francisco University, Brazil
3Professor of Dentistry at the University of Brazil, Brazil
4Microbiology Laboratory, Graduate Program in Health Sciences, São Francisco University, Brazil
Correspondence: Miguel Simão Haddad Filho, Professor of Dentistry at the University of São Francisco, Bragança Paulista- SP, Brazil
Received: December 24, 2022 | Published: March 31, 2023
Citation: Filho MSH, Rosa GP, De Oliveira MHS, et al. Comparative in vitro study of the disinfectant potential of three substances used in endodontics. J Dent Health Oral Disord Ther. 2023;14(1):20-26. DOI: 10.15406/jdhodt.2023.14.00589
To ensure the cleaning and sanitization of the root canal system during mechanical and chemical preparation, potentiation by physical agents is important. The literature shows that an average of 35% to 53% of the canal walls remain untouched, exposing the limitations of mechanical instruments and emphasizing the importance of chemical substances for chemical-surgical preparation and tubular decontamination. The aim of the present study was to analyze the disinfecting capacity of three chemical substances used in endodontic treatment on an aggressive species of microorganism. The methodology applied was an experimental laboratory study to compare the antimicrobial potential of 1% Sodium Hypochlorite, 2% Chlorhexidine and 17% Silver Nanoparticle, used in endodontics against the pathogen E. Faecalis, selected from the microorganism bank of the laboratory of Molecular and Clinical Microbiology of the Graduate Program in Health Sciences at and whose storage and use was previously authorized by the Research Ethics Committee of the São Francisco University. After collecting data, it was possible to check the results and conclude that the 2% chlorhexidine solution presented the best results in terms of antimicrobial efficacy compared to the 1% sodium hypochlorite solution, followed by the silver nanoparticles at 17%. The latter was not able to form a growth inhibition halo against E.faecalis in vitro.
Keywords: root canal, irrigants, disinfection
For proper disinfection, it is important to emphasize that millions of tubules beyond the root canal system (RCC) represent a huge web, and once installed necrosis is conducive to rapid colonization, proliferation, organization and destruction.1
Thus, the suspension of blood supply combined with the inability to respond to microbiological aggression, in addition to the favoring given by appropriate temperature, oxygen tension and especially the presence of nutrients represent an impediment to tissue reaction since dynamics of bacterial proliferation is organized around the polysaccharide matrix occupying entire interior of the canal and dentinal tubules, from there, also irritate the periradicular tissues.2
To ensure the sanitation of root canals one must include the use of instruments capable of excising and removing contaminated dentin aided Moreover, many techniques are proposed to ensure the sanitation of root canals potentiated or not by antimicrobial chemicals.3,4
Moreover, this can be done with continuous rotatory kinematics, alternating, oscillatory or hybrid. However, the dental surgeon must to know the characteristics of the instrument, form of use (mechanized rotary instrumentation).4,5
Limitations of mechanical instruments, associated with chemical substances, during root canal preparation and subsequent tubular decontamination showed that on average 35% to 53% of the canal walls remain untouched.6 It is a clear that the intra--canal medication after the preparation is less and less used considering to automated instrumentation allows treatments in a single session, because the control is given exclusively by the mechanical action of the instrument due to the high capacity to excise contaminated dentin and the contact of chemicals.4
Such preparation greatly reduces the bacterial load thanks to the action of antibacterial agents in dentinal tubules. Sodium hypochlorite (NaOCl), chlorhexidine (CHX) and silver nanoparticles (AgNPs) represent best choice in canal irrigation due to its antimicrobial action, useful to dissolve organic matter and cheap. As a downside toxicity when injected into the periapical ragion tissue, unpleasant smell and taste, affects the mechanical properties of dentin.7,8
Alternative use of chlorhexidine to sodium hypochlorite owing the antibacterial properties being able to avoid root canal reinfection. Although, it is not recommended to replace sodium hypochlorite as the main primary irrigant, as it does not have organic dissolution capacity and does not break down the biofilm.9
However, solutions that contain silver nanoparticles are an alternative in irrigation due to the biocompatibility and the high antibacterial potential because the silver ions are released thanks to oxidation after binding to the cell membrane and have the ability to damage the cytoplasm and bacterial DNA, causing the death of microorganisms.10
In order to alternative irrigating substances to sodium hypochlorite justifies conducting analysis whose goal is to confront the effects of chemical-surgical preparation in order to provide relevant data for scientific contribuition.
Vianna et al. ,11 inquire the antimicrobial effects of two types of solutions against endodontic bacteria, namely: 0.2%, 1% or 2% chlorhexidine gluconate (gel and liquid) and 0.5%, 1.0%, 2.5%, 4.0% or 5.25% sodium hypochlorite based on stock dilution test and the time for the irrigating to act against microbial cells. Both gel and liquid of 2.0% chlorhexidine eliminate Staphylococcus aureus and Candida albicans in 15 seconds, against Enterococcus faecalis, the gel was able to eliminate the pathogens in 1minute time while all Porphyromonas endodontalis, Porphyromonas gingivalis and Prevotella intermedia were eliminated within 15 seconds. Therefore, it was concluded that liquid chlorhexidine 1.0% or 2.0% and sodium hypochlorite 5.25%% were effective in eliminating all microorganisms at the same time.
Sena et al. ,12 tested the antimicrobial action both 2.5% and 5.25% sodium hypochlorite and 2.0% liquid and gel chlorhexidine on endodontic microorganisms such as Enteroccocus faecalis, Staphylococcus aureus, Candida albicans, Prevotella intermedia, Porphyromonas gingivalis, Porphyromonas endodontalis and Fusobacterium nucleatum deep in the substances during 30 seconds and still for 5, 10, 15, 30 and 60 minutes, with and without mechanical agitation and as a control group sterile saline. Based on the use of cellulose nitrate membrane inserted in agar medium in which biofilms of Enteroccocus faecalis, Staphylococcus aureus, Candida albicans, Prevotella intermedia, Porphyromonas gingivalis, Porphyromonas endodontalis and Fusobacterium nucleatum were stimulated. After that, the colony forming units were calculated the antimicrobial agents in liquid presentation, mainly sodium hypochlorite 5.25% and chlorhexidine 2%, showed faster reduction of the microorganism. All solutions eliminated the pathogens, with or without agitation, within 30 seconds while salinne solution did not inhibit the were able to eliminate the microorganisms faster. All solutions tested eliminated all pathogens, with or without agitation, within 30 seconds. The saline solution did not inhibit the growth of any pathogens. Chlorhexidine 2% and sodium hypochlorite 5, 25% effective in eliminating bacteria.
Davis et al.,13 investigated the antimicrobial action against Enteroccocus faecalis several solutions, namely: Dermacyn, BioPure MTAD, 2% chlorhexidine and 5.25% sodium hypochlorite. For this, 18 agar Petri plates were inoculated with the pathogen. In group I, 9 plates were incubated aerobically, and in group II, 9 plates were cultivated anaerobically at 37° C during for 48 hours. The effectiveness of the substances was calculated by the large diameter in millimeters of the microbial inhibition zones. Then results showed that BioPure MTAD showed significantly more zones of inhibition than 5.25% sodium hypochlorite, 2% chlorhexidine and Dermacyn. Sodium hypochlorite and chlorhexidine showed significantly more zones of microbial inhibition than Dermacyn. The difference between the zone of inhibition of sodium hypochlorite and chlorhexidine was not significant. It was concluded that BioPure MTAD was the solution that showed greater antimicrobial power, than sodium hypochlorite and chlorhexidine with similar results.
Ferraz et al.,14 evaluated the antimicrobial action of chlorhexidine gluconate gel compared to sodium hypochlorite using an agar diffusion test, in which the zones of progression inhibition produced by 0.2, 1.0% and 2.0% chlorhexidine and sodium hypochlorite were assessed beside 5 anaerobic bacteria and 4 Gram-negative pigmented anaerobes. Chlorhexidine 2% produced great zones of growth inhibition, and significantly greater than the zones of inhibition produced by all concentrations of 5.25% sodium hypochlorite. Chlorhexidine was feasible and safe showing effective antimicrobial properties against endodontic pathogens.
Sassone et al.,15 examined in vitro the antimicrobial action of sodium hypochlorite 1.0% and 5.0% and chlorhexidine 0.12%, 0.5% and 1.0% with or without added organic material, against endodontic pathogens Staphylococcus aureus, Enteroccocus faecalis, Escherichia coli, Porphyromonas gingivalis and Fusobacterium nucleatum using two contact and diffusion agar test. In the contact test, the bacterial samples were kept in contact with the tested irrigation solutions for different time intervals: 0, 5, 15 and 30 minutes. In the agar diffusion test, bacterial growth was evaluated for each microorganism and both repeated 10 times. In the contact test, the 0.12% chlorhexidine solution did not kill Enteroccocus faecalis at any time, while the 0.5% chlorhexidine solution killed all strains except Enteroccocus faecalis after immediate contact. The other solutions tested (chlorhexidine 1.0%, sodium hypochlorite 1.0% and sodium hypochlorite 5.0%) eliminated all strains. In the agar diffusion test, all solutions showed zones of antimicrobial action but the presence of organic material interfered in the antimicrobial action.
Pretel et al.,16 reviewed the antimicrobial action such as chlorhexidine and sodium hypochlorite in endodontics. An optimal irrigating solution should have good antimicrobial action, ability to dissolve tissue residues and good tissue biocompatibility. In general, sodium hypochlorite is used in different concentrations for root canal irrigation and chlorhexidine has been studied as an irrigating solution because of its antimicrobial properties and low toxicity. It was concluded that chlorhexidine in various concentrations was considered a viable alternative as an irrigation solution in endodontics, because it showed similar results to sodium hypochlorite. However, sodium hypochlorite in various concentrations is the most used irrigation solutions recognizes to its elevated ability to dissolve organic material and its better effect against Enteroccocus faecalis.
Gonçalves et al. ,17 detected trustworthiness of sodium hypochlorite and chlorhexidine in root canal disinfection of sodium hypochlorite and chlorhexidine in root canal disinfection. In 2 of the selected studies, both substances showed similar rates of reduction in bacterial levels. In two other studies, opposite results were observed, with one favoring the use as use sodium hypochlorite as the other chlorhexidine. In the last study evaluated, both irrigants were unable in eliminating necrotic root canals microorganisms.
Siqueira et al.,18 assessed the role of chlorhexidine and sodium hypochlorite solutions as irrigating endodontic treatment. Sodium hypochlorite shows excellent properties as an irrigant because it is able to promote good dissolution of organic material and a great antimicrobial action. Chlorhexidine has an excellent antimicrobial effect and a substantivity that prolongs its effect; however its power to dissolve organic material is reduced. With regard to the association of the two substances, it can be effective in terms of antimicrobial action, however, if it is not neutralized, it forms a precipitate capable of staining the root and forming a smear layer that is not water soluble being both effective and safe for endodontic use.
Rôças et al.,19 judged the antibacterial action of irrigants (sodium hypochlorite 2.5% and chlorhexidine 2.0%) in root canal preparation infected with Ni-Ti rotary instruments in 50 single-rooted teeth and apical periodontitis. Were divided equally into two groups: group I (sodium hypochlorite 2.5%) and group II (chlorhexidine 2.0%). Samples were obtained from the canal before treatment and after chemical-mechanical preparation. The analysis was done using DNA extracted from the clinical samples, and the reduction in levels of total bacteria and streptococci levels was evaluated using a quantitative polymerase chain reaction assay based on the 16S ribosomal gene. Before treatment, all samples were positive after the chemical-mechanical preparation, the sodium hypochlorite group showed 44% of infected canals versus 40% in the chlorhexidine group. In the total bacterial count, there was an indication that both substances were effective in decreasing the presence of bacteria, with no difference between them. There was no significant difference in the effectiveness of the clinical point of view of the two tested substances.
Samiei et al.,20 tested the use antimicrobial agent as nanoparticles in endodontic treatments in 15 studies selected in the review. The results indicated that silver nanoparticles (AgNPs) were the most studied agents thanks to their antimicrobial potential. Compared to bioactive glass nanoparticles and calcium derivative-based nanoparticles. They observed that bioactive non-organic nanoparticles in general showed effective and safe antimicrobial activity in treating root canal infections concluding that the use of nanoparticles showed similar or better results than conventional irrigants.
Haddad Filho21 investigated the contact disinfecting ability over a short period of time within the root canal system using system an automated system on 50 extracted human maxillary lateral incisors prepared with a single file (WaveOne Gold/ Primary/ Dentsply®) The roots were externally waterproofed, followed by contamination of the canals with E. Faecallis and subsequent centrifugation for tubular invasion. After colonization period, the samples were divided into 5 groups and submitted to disinfectant action, namely photodynamic therapy (PDT), silver nanoparticles (AgNPs), activated sodium hypochlorite (NaOCl 1%) sodium hypochlorite 1% and the control group with saline solution. It was concluded that sodium hypochlorite 1% with sonic activation was most effective in decontamination, followed by AgNPs. The sodium hypochlorite 1% in a short contact time did not present satisfactory results. PDT had a very unsatisfactory result, close to the control group.
Dal Bello et al.,22 evaluated the antimicrobial action of sodium hypochlorite and calcium hypochlorite irrigants the reciprocating system in endodontic treatment in 60 root canals in extracted human teeth, which inoculated Enteroccocus faecalis for 14 days. Were divided into 6 groups used: G1 no treatment, G2 distilled water, G3 sodium hypochlorite 2.5%, G4 calcium hypochlorite 2.5%, G5 sodium hypochlorite 5.25%, and G6 calcium hypochlorite 5.25%. The colony forming units were counted before and after procedure. Groups 1 and 2 had the highest mean contamination rates, with significant difference among them. Groups 3, 4, 5 e 6 had the lowest contamination rates, however, without statistical significance between both groups. It was concluded that the use of sodium hypochlorite or calcium hypochlorite, combined with reciprocating instrumentation, showed effective and similar antimicrobial action in the root canals infected with Enteroccocus faecalis.
Rodrigues et al.,23 performed the antimicrobial action of different irrigants as silver nanoparticles in aqueous vehicle, sodium hypochlorite and chlorhexidine on Enteroccocus faecalis biofilm which were inoculated in bovine dentin blocks for 21 days. After this the blocks it was irrigated with silver nanoparticles solution at 94 ppm, sodium hypochlorite at 2.5% or chlorhexidine at 2.0%, for three distinct times: 5, 15 or 30 minutes and evaluated by staining with the Live/Dead ratio technique using a confocal laser scanning microscope. The results indicated that the silver particles solution was the least efficient in eliminating bacteria; however, it promoted greater biofilm dissolution compared to chlorhexidina. Sodium hypochlorite had the highest antimicrobial activity and the best ability to dissolve biofilm.
Zandi et al.,24 considered the effectiveness of 2.0% chlorhexidine and 1.0% sodium hypochlorite as irrigating solution in 52 obturated teeth with apical periodontitis comprised in the study and distributed into 2 groups according to the irrigant used. Clinical and radiographic results were assessed, as well as the molecular aspect through real-time quantitative polymerase chain reaction (qPCR). Microbiological samples were obtained from the root canal system before treatment, after chemical-mechanical preparation, and after calcium hydroxide medication. At the 1-year follow-up, 45 teeth were open for analysis being 20 the sodium hypochlorite group and 25 the chlorhexidine group. It was found that 65% of the teeth sodium hypochlorite group and 64% chlorhexidine group healed. After follow-up 4 years, 33 teeth were available, being 16 the sodium hypochlorite group and 17 in the chlorhexidine group. It was found that 81% healing in the sodium hypochlorite group and 82% in the chlorhexidine group. The qPCR analysis indicated that canals that produced negative results after intracanal medication had a higher healing rate (79%) than positive canals (45%). It was concluded that no significant differences in clinical outcome between of the solutions.
Balto et al.25 collated the efficacy of different types of irrigating solutions against Enteroccocus faecalis. The solutions tested were calcium hydroxide, silver nanoparticles (AgNPs) and 1 mg/ml triple antibiotic paste (TAP). They inoculated Enteroccocus faecalis on dentin discs for 3 weeks. After this period, the samples were divided into groups containing 20 discs each, according to the substance used: group I, TAP 1 mg/ml; group II, calcium hydroxide and AgNPs mixture; group III, calcium hydroxide; group IV, AgNPs. Ten untreated dentin discs were exposed to sterile saline solution and left as a positive control, and ten sterile dentin discs as negative control and evaluated by staining with the Live/Dead ratio technique using a confocal laser scanning microscope. After 2 or 4 weeks, higher proportion occurred significantly higher number of dead cells in treated samples with 1 mg/mL TAP (90.39% to 99.41%) and in samples treated with the mixture of calcium hydroxide and AgNPs (90.85% to 98.49%) than in the samples treated with calcium hydroxide alone (76.14% to 91.71%) or AgNPs (62.83% to 88.07%). It was concluded that all the irrigating solutions used were effective in combating E. faecalis, with the mixture of calcium hydroxide and AgNPs showing a similar disinfecting effect to 1 mg/ml TAP.
Ruksakiet et al.26 compared antimicrobial action of chlorhexidine and sodium hypochlorite as irrigants in endodontic treatment. Clinical studies obtained from scientific databases were included (PubMed, EMBASE, Web of Science, and Cochrane Library). In all, 8 studies were selected to compose the systematic review. There was no significant difference between chlorhexidine and sodium hypochlorite regarding the change in the mean number of bacteria. It was concluded that both irrigating solutions are able to reduce bacteria, however, without any significant difference in antimicrobial efficacy between them.
Bhandi et al.,24 reviewed in PubMed, SCOPUS, Web of Science, and Embase scientific databases.the use of silver nanoparticles (AgNPs) as root canal irrigants. Only articles published in English were included, with no time restriction. After the evaluation of the available articles, 5 in vitro studies were included and selected to compose the review. According to the result of the analysis of the articles, it was observed that AgNPs have an antimicrobial effect to varying degrees, depending on factors such as the size of the particles used and the duration of contact possessing effective antibacterial action.
Tulu et al.27 researched in vitro the antibacterial action using silver nanoparticles (AgNPs) mixed with calcium hydroxide or chlorhexidine gel against a biofilm (Enteroccocus faecalis, Streptococcus mutans, Lactobacillus acidophilus and Actinomycesnaes lundii) on dentin blocks for 1 week. After this period, the blocks were divided into groups: group I, saline solution; group II, calcium hydroxide; group III, calcium hydroxide + silver nanoparticles (AgNPs); group IV, 2.0% chlorhexidine gel; and group V, 2.0% chlorhexidine gel + silver nanoparticles (AgNPs). Application times were 1 and 7 days and the bacterial samples were collected before and after application of the solutions to quantify the bacterial load by staining and confocal laser scanning microscopy (CLSM). The results pointed out that addition silver nanoparticles (AgNPs) to calcium hydroxide increased the antibacterial efficacy both application times (1 and 7 days). Addition of silver nanoparticles (AgNPs) to chlorhexidine was significantly efficient destruction of microorganisms when compared to all other solutions at all application times. It was concluded that the use of AgNPs as an adjuvant to calcium hydroxide or chlorhexidine irrigation solutions was effective in promoting greater antibacterial action.
Zhou et al.,28 evaluated the antimicrobial action of irrigating solutions (chlorhexidine and sodium hypochlorite) in 8 reviewed clinical studies. The main variables observed were the difference in number of bacteria the incidence of positive bacterial growth after irrigation. The results indicated that there was no significant difference in the incidence of positive bacterial growth or the mean number of bacteria between the chlorhexidine and sodium hypochlorite. Both substances with similar results in terms of disinfectant action.
Queiroz28 in a literature review compared the antimicrobial action of two irrigation solutions in endodontics: chlorhexidine and sodium hypochlorite. For this, Pubmed, LILACS and SCIELO databases were searched, and 15 articles were selected to compose the review. Of these, most (8) reported similar results for the antimicrobial action of chlorhexidine and sodium hypochlorite, with superior efficacy attributed to sodium hypochlorite in 5 studies, while 2 studies showed better performance of chlorhexidine both being effective antimicrobial actions.
Wang et al., reviewed the use of silver nanoparticles and its antibacterial properties in dentistry and that these materials have been researched due to their highly effective antimicrobial capacity associated with low toxicity. The antimicrobial capacity of silver nanoparticles is achieved by penetrating microbial cells membranes, causing damage to genetic material, death and dysfunction of bacterial proteins and enzymes. The use of silver has brought new perspectives in dentistry, and new applications have been developed caries prevention, root canal sterilization, inhibition of periodontal plaque, additives in prosthetics, coating of implants, and anti-inflammatory material in oral and maxillofacial surgery. Silver nanoparticles are a promising element during its use in dentistry due to its antimicrobial potential.
Medeiros et al.8 confirmed that mechanical instrumentation is increasingly efficient, and the rate of effective endodontic failure has decreased due to advancement in the introduction of auxiliary chemical substances for instrumentation such as sodium hypochlorite and chlorhexidine solution that offer the possibility of disinfecting the root canal system as well as the need for new protocols that must be performed in a single session. They proposed in this investigation, based on the pertinent literature of scientific articles searched in the Google and Academic PubMed databases to compare the action of two irrigating substances, sodium hypochlorite and chlorhexidine, both used in protocols from different colleges, one uses sodium hypochlorite solution because of its ability to dissolve tissues besides its antibacterial action (School of Dentistry, University of São Paulo) and the other uses chlorhexidine in gel or liquid form, as an irrigant (School of Dentistry, University of Campinas) which has the differential property of binding the entire dental substrate, being non-toxic to periodontal tissues and having a long-term antimicrobial action.
The aim of this research is to examine antibacterial action and disinfectant capacity, in vitro, of three chemical substances used in endodontic treatment namely: 2% Chlorhexidine, 1% Sodium Hypochlorite solution and 17% Silver Nanoparticles on a species E. faecalis.
Disc Diffusion Test
The antimicrobial activity of 2.0% chlorhexidine solution, 1.0% sodium hypochlorite solution and 17% silver nanoparticle solution against 10 clinical isolates of E. faecalis was evaluated using the diffusion test. These were obtained from the microorganism bank of the Microbiology Laboratory of the USF Graduate Program in Health Sciences, which were cultured in TSA medium (TrypticSoy Agar, Oxoid) for 24h during 37°C. Two consecutive replicates were performed before the experiments to ensure the purity and viability of the isolates.
Bacterial inoculate were prepared on McFarland's 0.5 scale in 0.75% saline solution and inoculated onto Mueller-Hinton agar plates (Oxoid) using a sterile swab. Over the inoculum, 4 sterile filter paper discs were added and 5 µL of each solution was dispensed onto the respective discs, as illustrated in Figure 1. One disk was used as negative control, on which, 5 µL of 0.75% Saline solution was added.
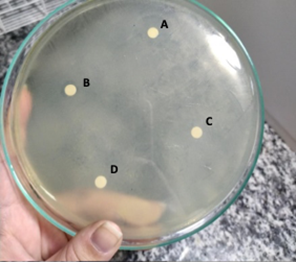
Figure 1 Arrangement of the antimicrobial solutions in the petri dish. A. Chlorhexidine 2% solution; B. Sodium hypochlorite 1% solution; C. Silver Nanoparticle Solution 17%; D. Negative Control (Saline 0.75%).
Source: own author (2022).
The plates were incubated at 37°C for 24 hours. After incubation, the diameter of the inhibition halos was read in millimeters for comparison between the solutions.
Microdilution broth test
The solution of Silver Nanoparticle 17% by means of the Diffusion Disc, the broth microdilution technique was used, to verify a possible difficulty in diffusing the solution in solid medium. From the pure solution of 17% Silver Nanoparticles, 10 serial dilutions were performed. One hundred microliters of each dilution were dispensed into a polystyrene microplate with a U-bottom, as illustrated in Figure 2.
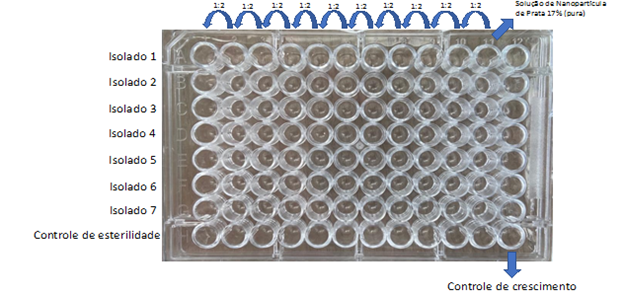
Figure 2 Illustration of the micro dilution plate in broth of the Silver Nanoparticle Solution. 17%. The pure solution was added in column 11 and serial dilution (1:2) was performed up to column 1. The bacterial isolates were added in rows A to G. Column 12 was used as a bacterial growth control and row H was used as a plate sterility control.
Source: authorship (2022).
The bacterial inoculum of the 10 clinical isolates of E. faecalis was prepared in 0.75% saline solution at a turbidity corresponding to McFarland's 0.5 scale (1.5 x 108). Sixteen microliters of this inoculum were diluted again in Mueller-Hinton broth to obtain a final volume of 5 mL, reaching a concentration of 1:32 (5 x 106). One hundred microliters of the bacterial inoculum were dispensed into the microdilution plate, as shown in Figure 2.
Column 12 was used as a growth control, so no Silver Nanoparticle solution was added. Row H, was used as a sterility control, so no bacterial inoculum was added.
The 2.0% chlorhexidine solution showed the highest antimicrobial activity against Enteroccocus faecalis isolates, followed by the 1.0% sodium hypochlorite solution. The inhibition halos were on average 50% larger for Chlorhexidine (Table 1).
Bacterial clinical isolate |
Diffusion Disc (halo of inhibition in mm) |
|
Chlorhexidine 2% |
Sodium Hypochlorite 1% |
|
861 |
10mm |
5mm |
886 |
10mm |
4mm |
993 |
13mm |
5mm |
994 |
9mm |
5mm |
1138 |
12mm |
5mm |
1142 |
10mm |
3mm |
1160 |
8mm |
5mm |
1181 |
10mm |
5mm |
1193 |
11mm |
5mm |
1227 |
8mm |
4mm |
Table 1 Measurements of the inhibition halos of the antimicrobial solutions by the disk diffusion technique
The 17% Silver Nanoparticle solution did not show a halo of inhibition for any clinical isolate of E. faecalis tested (Figure 3). For this reason, suggesting that the Silver Nanoparticle solution may not diffuse effectively in solid culture medium, it was retested using the broth microdilution technique.
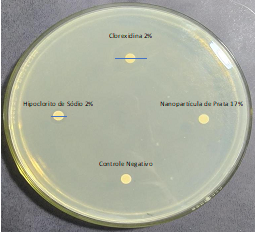
Figure 3 Inhibition halos formed by the antimicrobial solutions using the Disc Diffusion technique.
Source: own author (2022).
Using the broth microdilution technique, it was possible to observe that the bacterial isolates were not able to grow in the presence of the 17% pure Silver Nanoparticle solution. However, when diluted in culture medium, the solution was not able to inhibit the growth of E. Faecalis isolates (Figure 4).

Figure 4 Silver nanoparticle broth micro dilution result against E. faecalis isolates. Column 11 of both plates shows that no isolate was able to grow in the presence of the 17% pure silver nanoparticle solution. In columns 1 to 10, on the other hand, it is possible to observe the growth of the isolates (turbidity and bud at the bottom of the well) in all the most diluted concentrations.
Source: own author (2022).
As stated by Pretel et al.16 one of the main important aspects to be taken into consideration when choosing an irrigating solution is its antimicrobial action, as well as its ability to dissolve tissue residues, and tissue biocompatibility. Siqueira et al.18 stated that both chlorhexidine (CHX) and sodium hypochlorite (NaOCl) have an optimal antimicrobial effect, and the two substances are differentiated by the fact that CHX has a substantivity that prolongs its effect, while NaOCl has a greater power to dissolve organic material. Gonçalves et al.17 compared the efficacy of NaOCl and CHX, and concluded that there was insufficient evidence to affirm the greater efficacy of one substance over the other.
According to the results of the present research, it was observed that the 2% CHX generated the largest halos of growth inhibition of E. faecalis. Compared to the 1% NaOCl, the CHX produced halos on average 50% larger, indicating a greater antimicrobial activity. AgNPs at 17% were not able to generate growth inhibition halos.
When comparing these results with those presented in the literature, we observed that heterogeneous results were found.
In an in vitro research by Vianna et al.11 it was found that 2.0% CHX had similar antimicrobial effect to 5.25% NaOCl against Enteroccocus faecalis. Davis et al.13 observed that the difference between the zone of inhibition of 5.25% NaOCl and 2.0% CHX was not significant. Similarly, in vitro research by Sena et al.12 demonstrated that the antimicrobial activity of 5.25% NaOCl was similar to that of 2.0% CHX, so both solutions were effective destructions Enteroccocus faecalis. More recent in vitro research by Dal Bello et al.22 highlighted the efficacy of 2.5% 5 or 5.25% NaOCl against E. faecalis.
It is necessary to take into account that the concentration of the irrigating substance evaluated interferes with the results. In the present research, we used a concentration of 2% for CHX and 1% for NaOCl, while most in vitro researches used a concentration of 2% for CHX and 5.25% for NaOCl. This difference may explain why most in vitro studies pointed to similar results when comparing the substances, while our results pointed to the significant superiority of CHX. Thus, Sassone et al.15 observed that agar diffusion test, all concentrations of CHX and NaOCl produced zones of antimicrobial action, but larger inhibition halos were observed when higher concentrations of the solutions were used.
In contrast, the results of in vitro study by Ferraz et al.14 pointed out that 2% CHX produced the largest zones of growth inhibition and was significantly more effective than NaOCl at all concentrations, including 5.25%. In the work of Sassone et al.15 it was observed that 1% NaOCl and 1% CHX had similar effects in inhibiting Enteroccocus faecalis, but at lower concentrations CHX did not obtain the same efficacy, which highlights the resistance of this microorganism. Compared to our results, it can be seen that the concentration of 1% for CHX, although lower than that used in this research, showed high efficacy, being similar to the 1% NaOCl.
The in vivo studies analyzed presented different results from the present research and from Ferraz et al. (2007), and corroborated most of the in vitro research cited by pointing out that the comparison between NaOCl and CHX yielded similar results. Rôças et al.,19 (2016) indicated that 2.5% NaOCl and 2.0% CHX were equally efficient in chemical-mechanical preparation. Zandi et al., reported that there were no significant differences in clinical outcome between 1.0% NaOCl or 2.0% CHX in the treatment of teeth with apical periodontitis. It is noted that none of the in vivo studies used CHX at 1.0%, which was the concentration used in the present research.
Literature reviews also indicated that CHX and NaOCl showed similar results regarding their antimicrobial power against endodontic pathogens, such as the works done by Ruksakiet et al.,26 Zhou et al., and Queiroz.28
With regard to AgNPs, the present research observed that AgNPs presented significantly lower efficacy than NaOCl and CHX. A similar result was observed by an in vitro study by Rodrigues et al.23 in which it was found that the AgNPs solution was the least efficient in eliminating Enteroccocus faecalis compared to 2.5% NaOCl and 2.0% CHX.29
However, a review by Samiei et al.20 showed a different result by reporting that AgNPs have a high antimicrobial potential to be harnessed in endodontics, and showed similar or better results than the use of conventional irrigants. However, they require further studies on their toxicity. Recent reviews on the subject by Bhandi et al.243 and Wang et al., indicated that AgNPs have an effective antibacterial action.
In an in vitro study, Haddad Filho5 observed that 1% activated NaOCl was more effective in decontamination against E. faecalis, followed by AgNPs. The antibacterial effect of AgNPs was also found by an in vitro research done by Balto et al.25 in which the mixture of Ca(OH)2 and AgNPs showed a similar disinfectant action to 1 mg/ml TAP. Results in vitro study by Tulu et al.27 pointed out that AgNPs can be used as an adjuvant to Ca(OH)2 or CHX irrigating solutions to promote greater antibacterial action.
The results the study are somewhat agreement article by Medeiros et al.9 when reviewing new auxiliary active substances for instrumentation as sodium hypochlorite and chlorhexidine solutions both are used in faculty protocols, being one sodium hypochlorite and the other chlorhexidine.
From the results obtained, it was concluded that the 2% chlorhexidine solution showed greater in vitro antimicrobial efficacy against the pathogen E.faecalis, followed by sodium hypochlorite 1%; the 17% silver nanoparticle solution was not able to form a zone of growth inhibition against E. faecalis by disc diffusion technique; pure 17% silver nanoparticle solution was able to inhibit the growth of E. faecalis, however, diluted solutions with culture medium were not able to inhibit bacterial growth.
None.
The author declares no conflict of interest.

©2023 Filho, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.