Journal of
eISSN: 2373-4345


Research Article Volume 6 Issue 4
VTPCHEM PTY LTD, Australia
Correspondence: Tamara Perchyonok, VTPCHEM PTY LTD, Glenhuntly, Melbourne, 4215, Australia
Received: February 07, 2017 | Published: February 21, 2017
Citation: Perchyonok T. Bio-active denture soft liner materials from design to application: in vitro approach. J Dent Health Oral Disord Ther. 2017;6(4):101-105. DOI: 10.15406/jdhodt.2017.06.00206
Background: Denture soft liners are mainly used in order to decrease pain as well as improve compliance in patients who are not able to tolerate denture induced stresses. Soft liner materials are currently used as dynamic impression materials and also as adjuncts in prosthodontics for management of traumatized oral mucosa due to their accuracy and stability. The use of the soft liners as an addition to the acrylic denture and prolonged use has a significant problem which is accumulation and colonization of Candida albicansand other microorganisms which are a main cause of denture stomatitis.
Aim Due to our interest in design and evaluation of bio-active materials such as copaiba oil, Propolis (Red, Brazilian) and Propolis (Chilean, Rotterdam Laboratory) as the bio-active materials has been shown to be promising as a topical antifungal agent, with recent clinical data indicating efficacy in the treatment of oral candidiasis.1 The major advantages of natural medicinal plant extracts as antimicrobial agents include enhanced safety and stability without any side effects, which lack with both organic and inorganic antimicrobial agents. This in vitro study is undertaken with the aim to test the efficacy of the bio-active denture soft liner combined with Copaiba oil, Propolis (Red, Brasilian) and Propolis (Chilean, Rotterdam Laboratory) as well as combination of Chitosan/Copaiba oil, Chitosan/Propolis (Red) and Chitosan/Propolis (Chilean, Rotterdam Laboratories) by investigating several biomechanical as well as effectiveness the against Candida albicansgrowth.
Results: It has been reported that the bonding between resilient lining materials and denture base materials is affected by aging in water, the nature of the denture base material and the temperature. Resilient denture liners immersed in water leach out plasticizers and absorb water. These two mechanisms affect the denture compliance and dimensional stability. The results of the mechanical testing of resilient lining materials are important and help determining which materials have the better resistance under tensile or shear loading. The experiments are currently on the way in our laboratory to evaluate the tensile strength of the bio-active modified soft re-liner materials as well as asses the performance of the materials under thermocycling conditions. All the test samples gave an average inhibition zone larger than the tetracycline control disc, thereby confirming the antibacterial activity of the different bio-active containing combinations against Candida albicans. Using the Student's T-test (p<0.01), there was a significant difference between the rest of the samples when compared to each other and the positive control. The amount of bio-actives (such as Propolis (Brazilian, Red), Propolis (Chilean) and Copaiba oil) release in swelling media was analyzed after 1, 2, 24, and 96 h of immersion. The amount of bio-actives (such as Propolis (Brazilian, Red), Propolis (Chilean) and Copaiba oil) release in swelling media was analyzed after 1, 2, 24, and 96 h of immersion. The propolis release by polymeric systems usually occurs in two steps: the release of certain amounts of propolis in the first day of swelling as well as a prolonged release in some cases. A trend could be observed in all curves after 4 days of immersion: there was a high bioactive release in the initial hours and the cumulative release reached constant values up to 1 day of immersion. No prolonged release was observed. Nonetheless, the samples released more bioactive to PBS than to Solution pH 4.0, probably indicating that the propolis release can be influenced by the media pH.
Conclusion: The major advantages of natural medicinal plant extracts as antimicrobial agents include enhanced safety and stability without any side effects, which lack with both organic and inorganic antimicrobial agents. This in vitro study is undertaken and evaluated the efficacy of the bio-active denture soft liner combined with Copaiba oil, Propolis (Red, Brazilian) and Propolis (Chilean, Rotterdam Laboratory) as well as combination of Chitosan/Copaiba oil, Chitosan/Propolis (Red, Brazilian) and Chitosan/Propolis (Chilean, Rotterdam Laboratories) and demonstrated that the designed materials are suitable for further development of bio-active soft reliner materials.
Resilient soft liners combined with Copaiba Oil, Propolis (Red, Brazilian), Propolis (Chilean, Rotterdam Laboratories) as well as Chitosan/bio-active combinations have shown in vitro antifungal efficacy up to 60 days suggesting that the possibility of this bio-active modification for therapeutic use against denture stomatitis and possibly other oral infections without compromising the shear bond strength between the denture base material and the soft liner materials.
Denture soft liners are mainly used for therapeutic purpose in patients who are not able to tolerate denture induced stresses.1 Soft liner materials, though being used widely as dynamic impression materials and also as adjuncts in prosthodontic for management of traumatized oral mucosa, have few physical and microbiological disadvantages.2 One such major severe problem is colonization of denture surface by Candida albicansand other microorganisms, thereby causing denture stomatitis.2 The candida associated denture stomatitis is a common condition in complete denture wearers, characterized by generalized inflammation of the palatal mucosa covered by the denture.3 It is estimated to affect about 72% of this population.4 Denture induced stomatitis can be managed by either denture repair or replacement, prophylactic measures adopted by the patients and prescribing antifungal drugs.5–6 Biofilms of Candida on mucosal and inert surfaces such as dentures may contribute to therapeutic failure by modifying the susceptibility to antifungal agents.7 This treatment is complicated further in early and institutionalized patients with limitation of motor skills and special needs due to factors like loss of memory, difficulty in proper cleaning of the denture and following strict routine application of topical antifungal agent.8 Poor patient compliance due to need for frequent drug application and associated adverse effects could also result in recurrence of disease.7 These short comings have stimulated the development of other methods of drug elution, such as the incorporation of antifungal or antimicrobial agents with denture acrylic resin or with soft liners. A method of treatment by combining tissue conditioner and antifungal agents was suggested initially.9 After that several attempts have been made to incorporate different antifungal agents such as propolis,10 zeolite,11,12 chlorhexidine,3 Fluconazole,3 punica granatum,13 Nystatin,6,14 Itraconazole,6 Miconazole,15 Ketoconazole,15 Clotrimazole1 in the resilient liners with varying degree of success. Chitosan has been used as a wound dressing material due to its superior tissue- or mucoadhesive property, hemostatic activity, low toxicity, relevant biodegradability and anti-infection activity.16 Chitosan is a cationic polysaccharide and its adhesive properties are mainly based on ionic interactions with tissues or mucus layers. Due to our interest in design and evaluation of bio-active materials such as copaiba oil, Propolis (Red Brazilian) and Propolis (Chilean, Rotterdam Laboratory) as the bio-active materials has been shown to be promising as a topical antifungal agent, with recent clinical data indicating efficacy in the treatment of oral candidiasis.17 The major advantages of natural medicinal plant extracts as antimicrobial agents include enhanced safety and stability without any side effects, which lack with both organic and inorganic antimicrobial agents. This in vitro study is undertaken with the aim to test the effcacy of the bio-active denture soft liner combined with Copaiba oil, Propolis (Red Brasilian) and Propolis (Chilean, Rotterdam Laboratory) as well as combination of Chitosan/Copaiba oil, Chitosan/Propolis (Red) and Chitosan/Propolis (Chilean, Rotterdam Laboratories) by investigating several biomechanical as well as effectiveness the against Candida albicansgrowth.
The silicone soft liner selected was GC Reline Extra soft (GC Dental Industrial Corp. Tokyo, Japan). Copaiba oil, Propolis (Red Brasilian) and Propolis (Chilean, Rotterdam Laboratory) Chitosan/Copaiba oil, Chitosan/Propolis (Red) and Chitosan/Propolis (Chilean, Rotterdam Laboratories) and a combination of were used as supplied.
Methods
Sample preparation and testing: The silicone-based, resilient denture liners (GC Reline Extra soft) and 10% v/v copaiba oil, 10% v/v propolis (Red, Brazilian) and 10% v/v Propolis (Chilean, Rotterdam Laboratory). Copaiba oil, Propolis (Red Brasilian) and Propolis (Chilean, Rotterdam Laboratory) Chitosan/Copaiba oil, Chitosan/Propolis (Red) and Chitosan/Propolis (Chilean, Rotterdam Laboratories) were applied to a heat-polymerized denture base acrylic resin to assess the tensile and shear bond strengths at the liner- denture base resin interface as well as the failure mode after deboding. Eighty specimens per bond strength test (20 for each denture liner) were fabricated in moulds (8.0 cm x 1.0 cm x 0.2 cm) constructed using a conventional dental flasking technique. The water-stored were tested in shear strength in a universal testing machine (Instron, Australia) at a crosshead speed of 5 mm/min until fracture. The rupture peak load was recorded. The debonded surfaces were examined under X10 magnification to assess the failure modes, which were classified as adhesive, cohesive or mixed. Adhesive failure was considered the one at the liner/resin interface; cohesive failure if fracture occurred totally within the liner material; and mixed failure was assigned when both failure modes were observed.
Microbiology investigations of the bio-active: A type strain of Candida albicans(NCPF 3118), obtained from the American Type Culture Collection (Manassas, USA) was used as test bacterium for estimating the antibacterial activity of the bio-active soft liner. The antibacterial activity of the prepared chitosan hydrogels were tested using the standard Kirby-Bauer agar disc diffusion method.18 Five to 6 mm deep Muller-Hinton agar (Oxoid, Basingstoke, UK) plates were inoculated by streaking a standardized inoculum suspension that match a 0.5 McFarland standard and containing 107- 108 colony forming units/ml with a throat cotton swab. For each test sample 500µg of bio-active softliner was applied to a 6 mm diameter paper disc. The paper discs were placed on the inoculated Muller-Hinton agar medium and incubated at 37ºC for 24 hours. The diameter of the zones of growth inhibition was measured with a caliper. Each measurement was done in triplicate and the testing of each sample was repeated 3 times. The antibacterial efficacy of the bio-active softliner were compared to antibiotic sensitivity discs (Mast Laboratories, Merseyside UL) containing 30µg of nystatin per disc.
Bioactive release from the modified soft reliner materials: To analyze the bio-active release (Propolis (Red, Brazilian), Propolis (Chilean, Rotterdam Laboratories and Copaiba oil) based on the total phenolic concentration, the swelling media was analyzed after 1, 2, 24, and 96 h of immersion via UV–Vis spectrometer, from 300 to 800 nm, using polystyrene cuvettes.19 For quantification of the amount of Propolis released, a standard curve was created by diluting the original Propolis in isopropanol resulting in several aliquots of known concentration, which were then analyzed in the same wavelength range. The area of the peak of these aliquots (of known concentration of bio-additive) was calculated and used to compare with those of the bio-additive released by the samples.
Shear bond strength investigations
Shear bond strength means for the water stored are shown on Figure 1, respectively. Percent failure mode frequency after de-bonding for each tested condition is given on Table 1. It has been reported that the bonding between resilient lining materials and denture base materials is affected by aging in water, the nature of the denture base material and the temperature.20 Resilient denture liners immersed in water leach out plasticizers and absorb water. These two mechanisms affect the denture compliance and dimensional stability. The results of the mechanical testing of resilient lining materials are important and help determining which materials have the better resistance under tensile or shear loading. The experiments are currently on the way in our laboratory to evaluate the tensile strength of the bio-active modified soft re-liner materials as well as asses the performance of the materials under thermos cycling conditions.
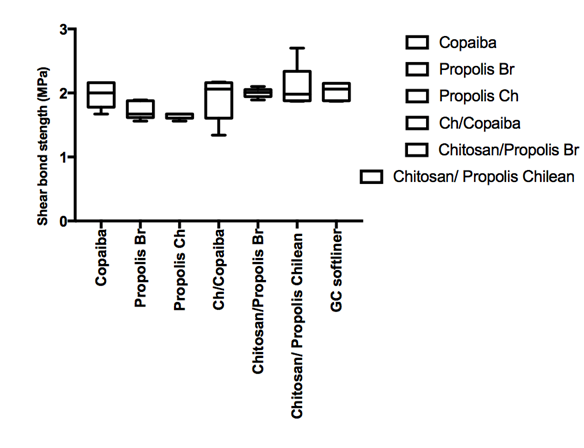
Figure 1 Shear bond strength in mPa for the GC soft liner material (standard versus the bio-active modified soft liner) and the denture base after 24 hours and materials were stored in artificial saliva solution at 37oC.
Material |
Bond strength test |
Adhesive |
Cohesive |
Mixed |
GC soft liner |
Shear |
20 |
60 |
20 |
Copaiba |
Shear |
20 |
60 |
20 |
Propolis (Brazilian, Red) |
Shear |
15 |
70 |
15 |
Propolis (Chilean) |
Shear |
20 |
60 |
20 |
Chitosan/Copaiba |
Shear |
20 |
60 |
20 |
Chitosan/Propolis Brazilian |
Shear |
20 |
60 |
20 |
Chitosan/Propolis Chilean |
Shear |
20 |
60 |
20 |
Table 1 Failure mode frequency (%) after de-bonding
Microbiological investigations
All the test samples gave an average inhibition zone larger than the tetracycline control disc, thereby confirming the antibacterial activity of the different bio-active containing combinations against Candida albicans(Figure 3).21 Using the Student's T-test (p<0.01), there was a significant difference between the rest of the samples when compared to each other and the positive control. The presence of Candida albicans in the denture stomatitis has been well documented in the literature and therefore development the bio-active soft liners can contribute to resolving the frequency and recurrence of the painful condition such as denture stomatitis and work is currently on the way in our laboratory to develop this material further.
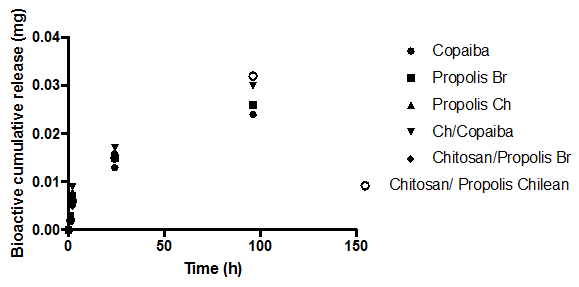
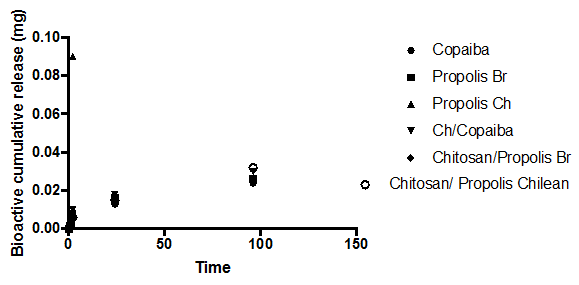
Bioactive release from the modified soft reliner materials
The amount of bio-actives (such as Propolis (Brazilian, Red), Propolis (Chilean) and Copaiba oil) release in swelling media was analyzed after 1, 2, 24, and 96 h of immersion (Figure 2A, 2b). The amount of bio-actives (such as Brazilian propolis, Propolis Chilean extract and Copaiba oil) release in swelling media was analyzed after 1, 2, 24, and 96 h of immersion (Figure 2A, 2B). The propolis release by polymeric systems usually occurs in two steps: the release of certain amounts of propolis in the first day of swelling as well as a prolonged release in some cases. 37 A trend could be observed in all curves after 4 days of immersion: there was a high bioactive release in the initial hours and the cumulative release reached constant values up to 1 day of immersion. No prolonged release was observed. Nonetheless, the samples released more bioactive to PBS than to Solution pH 4.0, probably indicating that the propolis release can be influenced by the media pH.
Bioadhesion and bioactive flowable materials
Higher adhesiveness of the gels is desired to maintain an intimate contact with skin or tooth structure and results are summarized in Table 2. Modified bioactive flowable composites showed the highest adhesive force and the work of adhesion this can be expected because of the well-known intrinsic bioadhesive properties of chitosan as well as propolis.22 The adequate water absorption capacity together with the cationic nature which promotes binding to the negative surface of skin or enamel structure can also interpret this result. The presented values are an average (n=5) Chitosan hydrogels showed the highest adhesive force and the work of adhesion this can be expected because of the well known intrinsic bioadhesive properties of chitosan. The detailed investigation of the newly produced bio-materials with particular attention being paid towards understanding of the exact nature of interaction between bio-actives such as propolis, chitosan and oral mucosa structure are currently on the way in our laboratory.
Bio-active soft |
Adhesive force (N)±SD |
Work of adhesion |
Standard |
1.12±0.35 |
3.45 ±0.30 |
Copaiba |
1.21±0.47 |
3.92±0.46 |
Propolis(Red, Brazilian) |
1.13±0.40 |
3.44±0.29 |
Propolis (Chilean) |
1.09±0.24 |
3.45±0.34 |
Chitosan/Copaiba |
1.18±0.40 |
3.89±0.44 |
Chitosan/Propolis (red) |
1.09±0.24 |
3.45±0.34 |
Chitosan/Propolis (Chelian) |
1.18±0.40 |
3.89±0.44 |
Table 2 Bioadhesion testing in vitro
Discussion of the anti-microbial properties of the soft liner materials
Removable prosthesis, when placed in the oral cavity produces numerous changes in the oral environment, which may adversely affect integrity of oral tissues, denture stomatitis being one of the important clinical presentations of oral candidiasis.23 Though the aetiology is multifactorial,24 denture biofilm components, such as C.albicans play a basic role in development of candidiasis.25 Newer agents from natural resources are required, which can inhibit the growth of microorganisms in the bio lm, and would enhance the effective alternative therapeutic modalities, as the action of antifungal agents may be limited by their penetration and chemical reaction into bio lm matrix, the extracellular polymeric material.26 Recently, incorporating extracts of medicinal plants into biomaterials have been in practice and found to be a natural alternative with excellent antifungal effects.27 This present study incorporated Copaiba oil, Propolis (Red, Brazilian) and Propolis (Chilian, Rotterdam Laboratory). Copaiba oil, Propolis (Red Brasilian) and Propolis (Chilean, Rotterdam Laboratory) Chitosan/Copaiba oil, Chitosan/Propolis (Red) and Chitosan/Propolis (Chilean, Rotterdam Laboratories) into silicone soft liner and evaluated its efficacy against growth of C. albicans. Results of present study suggested that treated disks Copaiba oil, Propolis (Red, Brazilian) and Propolis (Chilian, Rotterdam Laboratory) showed significant antifungal efficacy against C. albicanscompared to untreated disks upto 60 days, and this was in agreement with Al Mashhadane et al.28 showed that 15% tea tree oil had significant antifungal effect against C.albicans on the surface of heat cure acrylic denture base material. This study immersed the denture in tea tree oil for 24-48 hours instead of adding it in the denture itself. Our study has added tea tree oil into the soft liner so that there is continuous sustained release of Copaiba oil, Propolis (Red, Brazilian) and Propolis (Chilean, Rotterdam Laboratory) exhibiting antifungal activity up to 60 days, avoiding other alternative mechanical and chemical denture cleansing methods.29 We are currently investigating the detailed mechanism and incorporation of antimicrobial agent incorporation into the soft liner structure and results will be published in due course.
Resilient soft liners combined with Copaiba Oil, Propolis (Red, Brazilian), Propolis (Chilean, Rotterdam Laboratories) as well as Chitosan/bio-active combinations have shown in vitro antifungal efficacy up to 60 days suggesting that the possibility of this bio-active modification for therapeutic use against denture stomatitis and possibly other oral infections without compromising the shear bond strength between the denture base material and the soft liner material.
None.
None.
The authors declare that there is no conflict of interest.

©2017 Perchyonok. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.