Journal of
eISSN: 2574-9943


Case Report Volume 9 Issue 2
Altruva Aesthetic Clinic, Indonesia
Correspondence: Olivia Aldisa, Altruva Aesthetic Clinic, Indonesia, Tel +62 813 2133 0788
Received: April 25, 2025 | Published: May 15, 2025
Citation: Aldisa O. A case report on the comparative efficacy of a novel high-intensity parallel beam ultrasound device with and without liquid type polycaprolactone in treating facial wrinkles. J Dermat Cosmetol. 2025;9(2):45-48. DOI: 10.15406/jdc.2025.09.00290
This case report evaluates the efficacy of a high-intensity parallel beam ultrasound device alone versus its combination with liquid type polycaprolactone (PCL) for facial wrinkle treatment. The study aims to determine whether the combined approach enhances outcomes through synergistic effects on multiple tissue layers.
A case study was conducted, with the left side treated using ultrasound alone and the right side treated with ultrasound plus liquid type PCL. The ultrasound treatment involved 300 lines (150 per side) at 3.4 J. On the right side, 1 cc of liquid type PCL was injected subdermally. Assessments were conducted at baseline, day 14, day 30, and day 90 using the Modified Fitzpatrick Wrinkle Scale, Allergan Infraorbital Scale, and Global Aesthetic Improvement Scale.
By day 90, the combination treatment improved MFWS from 1.5 to 0 and AIS from 2 to 0, while ultrasound alone improved MFWS to 1 and AIS to 1. Investigator and patient GAIS ratings confirmed greater and faster improvements on the right side, with the patient reporting enhanced skin texture, brightness, and finer pores.
The combination of ultrasound and liquid type PCL resulted in significantly greater improvements in nasolabial wrinkle severity and infraorbital volume loss compared to ultrasound alone, suggesting a synergistic effect.
Keywords: high-intensity parallel beam ultrasound, liquid type polycaprolactone, PCL biostimulator, facial rejuvenation, wrinkle improvement
An increasing interest in aesthetic procedures performed in clinical settings, aimed at boosting collagen synthesis, has been noted.1 Enhancements in one's appearance through a tailored and multi-faceted strategy for facial aesthetic rejuvenation can lead to greater self-esteem and an improved quality of life.2 While various treatment modalities have been explored, the combination of energy-based devices and collagen biostimulators has emerged as a promising approach.3
Ultrasound technology, particularly micro-focused ultrasound (MFU), non-invasively targets subcutaneous tissue by raising temperatures to just above 60°C, creating small thermal coagulation points (less than 1 mm³) that penetrate up to 5 mm deep, leading to collagen denaturation, contraction, and stimulation in the SMAS, platysma, and deep reticular dermis for skin tightening.4
However, the previous ultrasound (US) devices for skin tightening could potentially cause significant epidermal damage, pain, and nerve injury due to limited energy discharge and deep skin penetration. The novel US device using Synchronous Ultrasound Parallel Beam (SUPERB™) Technology, with a 1.5 mm transducer and integrated cooling system, delivers high energy (up to 5 J) while preventing thermal damage, offering safer and more effective skin tightening.5,6
Polycaprolactone (PCL) is a biocompatible, biodegradable polymer that promotes long-term skin improvement by stimulating collagen, elastin, and neovascularization, with clinical studies showing its efficacy and sustained effects up to two years post-treatment.7
On the contrary, the drawbacks of conventional PCL injectables as a collagen biostimulator include technical challenges from PCL microparticles blocking the needle, uneven distribution and potential risk of overcorrection.8 Serious adverse events related to conventional PCL-based fillers are rare but can include complications such as infections, nodules, granulomas,9,10 and, in severe cases, vascular issues that may result in tissue damage or vision loss.
Novel liquid type polycaprolactone (GOURI, DEXLEVO Inc., Seoul, Korea) is a PCL-based collagen biostimulator that stimulates collagen production for facial rejuvenation with its liquid state, unique composition, and superior spreadability, offering a general lifting effect, fewer injection points, and enhanced skin elasticity through a three-dimensional matrix that promotes natural collagen regeneration.8,11 Additionally, recently published study suggests that the safety profile of the given injectable is enhanced compared to other conventional biostimulators, mentioning only mild-to-moderate adverse effects.12
This case report aims to investigate the comparative efficacy of a novel high-intensity parallel beam ultrasound device with and without the addition of a novel liquid type polycaprolactone in the treatment of facial wrinkles.
This case report describes a single-case study in which the left side of the face was treated with a novel high-intensity parallel beam ultrasound device alone, while the right side received the same ultrasound treatment combined with liquid-type polycaprolactone during the same session. Given the single-case approach and negligible-risk study design, the patient was thoroughly informed about the procedure and provided with a clearly outlined consent form covering both the treatment and the publication of results, including clinical photographs. A guarantee was also given regarding the eventual treatment of the initially untreated side. The study was designed and conducted in accordance with the ethical principles of the Declaration of Helsinki.
The technique used in the high-intensity parallel beam ultrasound device involves 300 lines, 150 lines per side, with an average energy of 3.4 J using the Thermal Thread Technique™.13 After the ultrasound, a treatment with liquid type polycaprolactone was conducted on the right side of the face using a 25G, 50mm cannula. For the treatment a total of 1 cc of product was used, divided into 4 lines (0.25 cc/line), administered subdermally with a retrograde linear technique, in accordance with commonly used protocols (Figure 1).14 Due to its liquid form the given biostimulator does not possess the risk of nodule formation, thus can be injected subdermally for a better synergistic effect. Prior to the procedure, the patient received 1cc of lidocaine infiltration based on the marked pattern.
The participants were assessed at days 7, 14, and 30, using the The Modified Fitzpatrick Wrinkle Scale15 and the Allergan Infraorbital Scale for volume changes.16 Investigator’s Global Aesthetic Improvement Scale (GAIS), Subject’s GAIS, and patient’s own evaluation were also recorded.
The rationale for this treatment approach is supported by the findings of recent studies, which have demonstrated the synergistic effects of combining energy-based devices, such as microfocused ultrasound, with collagen biostimulators like calcium hydroxyapatite and poly-L lactic acid in improving skin aesthetics and addressing wrinkles.1,3,17,18
It was hypothesized that the treatment on the right side, combining the ultrasound device with liquid type polycaprolactone, would produce more effective and sustained results compared to the left side, which was treated with the ultrasound device alone, due to the synergistic effect from doubled stimulation with liquid type polycaprolactone and US device.
The treatment results were assessed by the investigator using The Modified Fitzpatrick Wrinkle Scale (MFWS) and the Allergan Infraorbital Scale (AIS) (Figure 2 & Table 1). On the left side, treated with the ultrasound device alone, the wrinkle scale improved from a baseline of 1.5 (visible wrinkle and clear indentation, <1 mm wrinkle depth) to a score of 1 (fine wrinkle, visible wrinkle and slight indentation) by day 90 and the infraorbital scale improved from grade 2 (defined skin crease and mild volume loss) to grade 1 (subtle skin crease with mild volume loss). On the right side, treated with the ultrasound device and liquid type polycaprolactone, the wrinkle scale improved from a baseline of 1.5 to a score of 0 by day 90, and the infraorbital scale improved from grade 2 (defined skin crease and mild volume loss) to grade 0 (no visible skin crease and no visible volume loss) by the same time point (Figure 3 & Figure 4). It should also be noted that changes on the combination treatment side showed faster improvements.
|
Day |
MFWS (Left) |
MFWS (Right) |
AIS (Left) |
AIS (Right) |
|
Baseline |
1.5 |
1.5 |
2 |
2 |
|
Day 14 |
1.5 |
1 |
2 |
1 |
|
Day 30 |
1.5 |
0.5 |
2 |
1 |
|
Day 90 |
1 |
0 |
1 |
0 |
Table 1 Evaluation results in Modified Fitzpatrick Wrinkle Scale (MFWS) score and Allergan Infraorbital Scale (AIS) from baseline for each visit after the treatment with ultrasound only, and with ultrasound and liquid PCL combination.
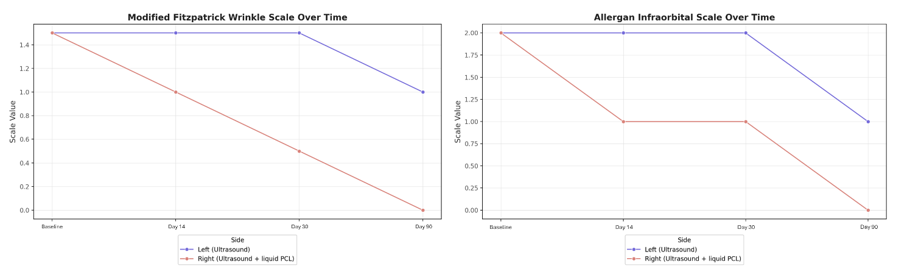
Figure 2 Change comparison in Modified Fitzpatrick Wrinkle Scale score and Allergan Infraorbital Scale from baseline for each visit after the treatment with ultrasound only, and with ultrasound and liquid PCL combination.

Figure 3 Photos of the patient (left side) at baseline, 30 days and 90 days after the treatment with ultrasound only.

Figure 4 Photos of the patient (right side) at baseline, 30 days and 90 days after the treatment both with ultrasound and liquid PCL.
The Investigator GAIS on day 30 showed a rating of 3 (improved) for the left side and a rating of 1 (very much improved) for the right side. Meanwhile, the patient GAIS showed a rating of 2 (much improved) for the left side and a rating of 1 (very much improved) for the right side.
Based on the patient’s self-evaluation, they felt that the right side showed faster improvement, particularly in the smile lines and under-eye area. The patient also noted smoother skin texture, brighter skin tone, and finer pores on the right side (Figure 5).
The results indicate that the treatment on the right side, utilizing the combination of the ultrasound device and liquid type polycaprolactone, demonstrated faster, more effective and significant improvements in both nasolabial wrinkle severity and infraorbital volume loss compared to the left side treated with the ultrasound device alone. Additionally, although the injection of liquid type PCL involved using only four lines as described in the methods section, the improved results were observed in the periorbital area, smile lines, and nasolabial folds suggesting the advanced spreadability of the product allowing for synergistic effect across the entire area treated with US.
The findings of this case report are consistent with the existing literature, which suggests that the combined use of energy-based devices, such as microfocused ultrasound, and collagen stimulators, including calcium hydroxyapatite and poly-L-lactic acid, can lead to more pronounced and sustained improvements in various skin aesthetic outcomes, including the reduction of facial wrinkles and the enhancement of facial volume.1,3,17
The addition of liquid type polycaprolactone to ultrasound device treatment on the right side of the face may have enhanced the superior results observed in this case report. Polycaprolactone, a biodegradable synthetic polymer, acts as a collagen stimulator that promotes new collagen production, improving skin texture and elasticity.8,12 Furthermore, unlike traditional collagen stimulators, liquid type polycaprolactone offers a more convenient alternative with significantly fewer side effects, ensuring a more even distribution, overcoming dilution-related challenges, and minimizing the risk of nodules or inflammation.10,11
The combination of high-intensity parallel beam ultrasound technology and the collagen stimulating properties of liquid type polycaprolactone likely created a synergistic effect by targeting the mid-dermis5,19 and deep dermis layers,11 respectively. Ultrasound-induced heat energy partially denatures collagen, triggering new collagen and elastin production,5 while the CESABP technology of liquid type PCL ensures uniform distribution in the skin without particles aggregation, activating fibroblasts through a distinct pathway to initiate neocollagenesis.8,11 As liquid type PCL does not stimulate nodule formation, at the moment it’s the only biostimulator that can be injected into superficial skin layers and used in combination protocols like this one to offer better synergistic effect when applied with ultrasound technology.
These combined mechanisms resulted in more comprehensive and effective improvements in nasolabial wrinkle severity and infraorbital volume loss compared to ultrasound treatment alone.
This case report demonstrates the potential benefits of combining a novel high-intensity parallel beam ultrasound device with a novel liquid type polycaprolactone in the treatment of facial wrinkles. The results suggest that this combined approach may be more effective than the ultrasound device alone in improving nasolabial wrinkle severity and infraorbital volume loss, as it provides a synergistic effect by targeting multiple tissue layers, leading to faster and more pronounced outcomes.
Further research with larger sample sizes and longer follow-up periods is required to validate these findings and explore the underlying mechanisms responsible for the observed improvements.
None.
The author declares there is no conflict of interest.
None.

©2025 Aldisa. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.