Journal of
eISSN: 2373-633X


Research Article Volume 16 Issue 2
1Department of Gastrointestinal Surgery, The Affiliated Shaoyang Hospital, Hengyang Medical School, University of South China, Shaoyang, Hunan, China
2Department of Pathology, The Affiliated Shaoyang Hospital, Hengyang Medical School, University of South China, Shaoyang, Hunan, China
Correspondence: Bo Wen, Department of Gastrointestinal Surgery, The Affiliated Shaoyang Hospital, Hengyang Medical School, University of South China, Shaoyang, Hunan, China
Received: April 21, 2025 | Published: May 1, 2025
Citation: Wang H, Liu S, Xie S, et al. The correlations between 91 inflammatory cytokines and malignant neoplasms of the stomach: an exploratory mendelian randomization analysis. J Cancer Prev Curr Res. 2025;16(2):36‒43. DOI: 10.15406/jcpcr.2025.16.00575
Numerous studies have suggested a possible link between inflammatory cytokines and gastric malignancies; however, the causal relationships remain ambiguous. To address this uncertainty, the present study utilized Mendelian randomization (MR) to investigate the causal effects of inflammatory cytokine levels on the development of malignant gastric neoplasms. Genetic variation datasets for 91 inflammatory cytokines were collected from genome-wide association studies (GWASs), and information on malignant neoplasms of the stomach was obtained from the Finnish database. The primary analysis was carried out via the inverse variance weighted (IVW) method. To evaluate the robustness and validity of the causal inferences, pleiotropy residual sum and outlier tests, MR-Egger intercepts, Cochran's Q tests, and leave-one-out analyses were conducted. Additionally, we utilized reverse MR to examine the possibility of reverse causation. The IVW results indicated that the levels of interleukin-10 receptor subunit beta (IL-10RA) (OR = 1.1468; 95% CI: 1.0064-1.3068; P = 0.0398) and the levels of tumor necrosis factor ligand superfamily member 14 (OR = 1.1693; 95% CI: 1.0016-1.3651; P = 0.0476) increased the likelihood of a high risk of malignant neoplasm of the stomach, whereas matrix metalloproteinase-1 (MMP-1) had a protective effect on the malignant neoplasm of the stomach (OR = 0.7813; 95% CI: 0.6125-0.9968; P = 0.0471). IL-10RA and MMP-1 were also validated in a subset of adenocarcinomas and papillary adenocarcinomas of the stomach. TNFSF14 and PD-L1 revealed positive evidence to support causality with malignant neoplasms of the stomach and adenocarcinoma, respectively. This study reveals potential associations between 91 inflammatory cytokines and the risk of malignant gastric neoplasms, as indicated by MR analysis. These findings provide important insights that may facilitate the development of diagnostic biomarkers and aid in the identification of novel therapeutic targets for gastric cancer.
Keywords: inflammatory cytokines, gastric malignant neoplasm, gastric adenocarcinoma, MR
MR, mendelian randomization; TSMR, two-sample mendelian randomization; GWAS, genome-wide association study; IV, instrumental variable; IVW, inverse variance weighted; MR-PRESSO, pleiotropy residual sum and outlier; SNP, single-nucleotide polymorphism; LD, linkage disequilibrium; OR, odds ratio; CI, confidence interval
Malignant neoplasms of the stomach pose a significant global health challenge and are characterized by high morbidity and mortality rates. According to the most recent GLOBOCAN database from 2022, gastric cancer accounts for approximately 4.9% of all cancer cases and is responsible for 6.8% of cancer-related deaths; projections suggest that these figures may change due to population growth and aging by 2050.1,2 Despite available treatment options, including radiotherapy, chemotherapy, surgery, and their combination, the prognosis for patients diagnosed with advanced-stage gastric cancer remains unfavorable.3 Cytokines are critical for mediating inflammatory interactions between immune cells and tumors during multiple stages of tumor development, including initiation, promotion, malignant transformation, invasion, and metastasis. Consequently, targeting cytokines represents a promising strategy for precision oncology.4
Chronic inflammation activates the immune system, resulting in elevated circulating cytokine and chemokine levels. Research indicates that chronic inflammatory states may heighten the risk of developing malignant gastric neoplasms,5,6 indicating that approaches focused on preventing or alleviating inflammation might help lower this risk. Inflammatory cytokines are essential for the initiation and progression of gastric cancer through multiple mechanisms and are increasingly recognized as both diagnostic markers and potential therapeutic targets. Inflammatory cytokines play crucial roles in the initiation and progression of gastric cancers through various mechanisms and are increasingly recognized as both diagnostic indicators and potential therapeutic targets.7–9 Future efforts should focus on identifying specific inflammatory cytokines linked to malignant gastric neoplasms, understanding their relationships with these cancers, clarifying the associated signaling pathways and improving survival outcomes for cancer patients.
However, the inflammatory processes occurring in the human body may differ from those observed in cellular or animal models. Furthermore, observational clinical studies can be influenced by various factors, such as differences in study designs, insufficient sample sizes, confounding variables, and population diversity along with concerns such as reverse causation. These factors complicate the establishment of a clear causal link between inflammatory cytokines and malignant gastric neoplasms. To address these challenges, the Mendelian randomization (MR) method employs single-nucleotide polymorphisms (SNPs) as instrumental variables (IVs) for causal inference.10 This approach helps alleviate the influence of confounding factors and reverse causation while also decreasing the time and financial costs of research. As a result, we conducted a two-sample MR analysis in this study to investigate the associations between 91 inflammatory cytokines and the risk of malignant gastric neoplasms.
Research framework
We performed a bidirectional two-sample MR study to explore the causal relationships between malignant gastric neoplasms and inflammatory cytokines, using SNPs as IVs. Figure 1 provides an overview of the MR framework. This study was conducted in compliance with the STROBE-MR standards. (Supplementary Table S1).11 The three primary assumptions of instrumental variables in MR analysis are as follows: (1) a strong association with the exposure variable (2) independence from confounding factors and (3) the instrumental variables affect the outcome exclusively through the exposure variable, without any influence from alternative pathways.12 Since we reanalyzed previously collected and published GWAS datasets that received approval from the relevant review boards no additional ethical approval was required from an ethics committee.
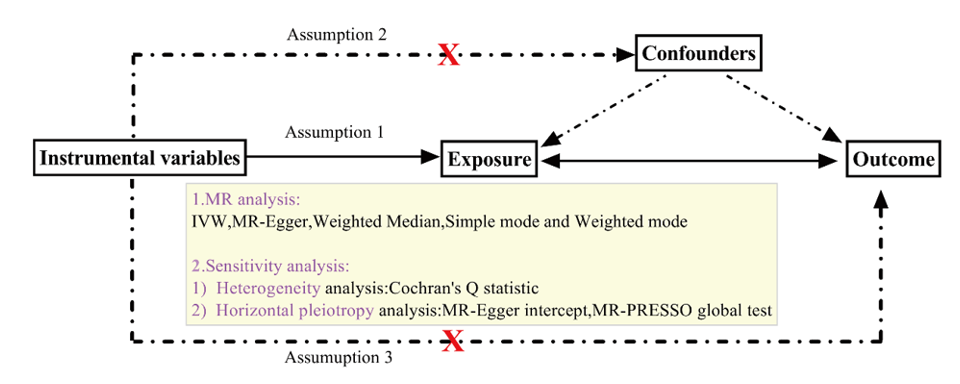
Figure 1 Study overview. IVW, inverse variance weighted; MR-PRESSO, pleiotropy residual sum and outlier.
Source of data
GWAS data for malignant neoplasms of the stomach
The summary data for gastric neoplasms were retrieved from the R11 GWAS dataset of the Finn Gen Consortium. This dataset comprises 1,741 cases of malignant neoplasms of the stomach and 345,118 controls, along with 956 cases of adenocarcinoma and papillary adenocarcinoma of the stomach, using the same control group. The data for this study were obtained from Finn Gen (https://www.finngen.fi/en/access_results).13
GWAS data of inflammatory cytokines
The genetic information on approximately 91 plasma inflammatory proteins was collected from the largest GWAS meta-analysis, released in 2023, with research accession numbers GCST90274758 to GCST90274848. This investigation included 11 groups of 14,824 people of European ancestry.14 The data utilized in this study were obtained from the EBI GWAS Catalog (https://www.ebi.ac.uk/gwas/). A summary of these data can be found in Supplementary Table S2.
Selection of instrumental variables
To enhance the selection of SNPs that exhibit significant associations with inflammatory cytokines and gastric neoplasms as potential instrumental variables, we initially applied a less strict genome-wide significance threshold to expand the SNP pool (P < 1E-05).15–17
To minimize linkage disequilibrium (LD) and ensure separate IVs, we used a clumping criterion (r2 < 0.001 within a 10000-kb region).18–21 Incompatible and palindromic SNPs were left out since it was unable to determine whether the IV was consistently aligned during the harmonization of exposure and outcome data. To reduce bias from weak instrumental factors, we calculated the F value for each SNP via the equations F = beta2 exposure/se2 exposure and R2 = 2 × (1 - MAF) × MAF × beta2, excluding SNPs with F values below 1022,23 Additionally, the Pheno Scanner database (http://www.phenoscanner.medschl.cam.ac.uk/) was used to detect potential pleiotropic confounders.24
Statistics
We conducted an MR analysis via the "Two Sample MR" package (version 0.5.6) and R (version 4.4.1) to examine the connection between exposures and outcomes.25 We evaluated the reliability of our data via five distinct methods: inverse variance-weighted random effects (IVW), MR-Egger, weighted median, simple mode, and weighted mode. The IVW method was our main analysis technique.26 To examine heterogeneity, we conducted a Cochran's Q test as part of a sensitivity analysis.27 The results of this heterogeneity assessment are represented through funnel plots of the instrumental variables. We also conducted MR pleiotropy residual sum and outlier (MR-PRESSO) tests, as well as MR-Egger regression tests, to detect any horizontal pleiotropic effects via the MR-PRESSO R package (version 1.0).28,29 Following the removal of pleiotropic SNPs, the remaining SNPs were employed for the subsequent MR analysis. A leave-one-out analysis was conducted to evaluate whether any single SNP affected the MR outcomes. All the statistical evaluations were carried out via a two-sided method with P values less than 0.05 considered statistically significant for this exploratory research.
Instrumental variable selection
In the MR analyses, we established a significance threshold of P < 1E-05 to ensure an adequate number of SNPs. Consequently, we selected 80 SNPs associated with 91 inflammatory cytokines related to malignant neoplasms of the stomach and 126 SNPs for adenocarcinoma and papillary adenocarcinoma of the stomach after excluding palindromic, ambiguous, and unproxied SNPs from the dataset. Additionally, all reported F statistics were greater than 10. These 206 SNPs were chosen as instrumental variables (IVs) for the 91 inflammatory cytokines (see Supplementary Table S3A-SB).
Forward MR analysis of the associations of 91 inflammatory cytokines with malignant neoplasms of the stomach
Our main analysis employed the IVW method, whereas MR-Egger, weighted median, simple mode, and weighted mode were used as complementary approaches. The IVW analysis (Figure 2) revealed a causal relationship between the levels of interleukin-10 receptor subunit beta (IL-10RA) and malignant neoplasms of the stomach (OR = 1.1468; 95% CI: 1.0064-1.3068; P = 0.0398), matrix metalloproteinase-1 (MMP-1) levels (OR = 0.7813; 95% CI: 0.6125-0.9968; P = 0.0471), and tumor necrosis factor ligand superfamily member 14 (TNFSF14) levels (OR = 1.1693; 95% CI: 1.0016-1.3651; P = 0.0476), and MMP-1 reduced the incidence of malignant neoplasms of the stomach. In the MR analysis concerning malignant neoplasms of the stomach, the Q tests for these three inflammatory cytokines indicated no evidence of heterogeneity, as reflected by Cochran's Q P values exceeding 0.05 in the IVW analysis. Moreover, no notable intercepts were identified, which supports the lack of pleiotropy (MR-PRESSO global test P value > 0.05) (Supplementary Table S4). Furthermore, the 'leave-one-out' analysis reinforced the robustness and credibility of our results, as no individual SNP exhibited a significant effect on the outcomes (Supplementary Figure S1A-S1C). We illustrated the results of the MR analyses in a consolidated forest plot (Figure 2) along with related forest plots (Supplementary Figure S2A-S2C), scatter plots (Figure 3A-3C), and funnel plots (Supplementary Figure S3A-S3C).
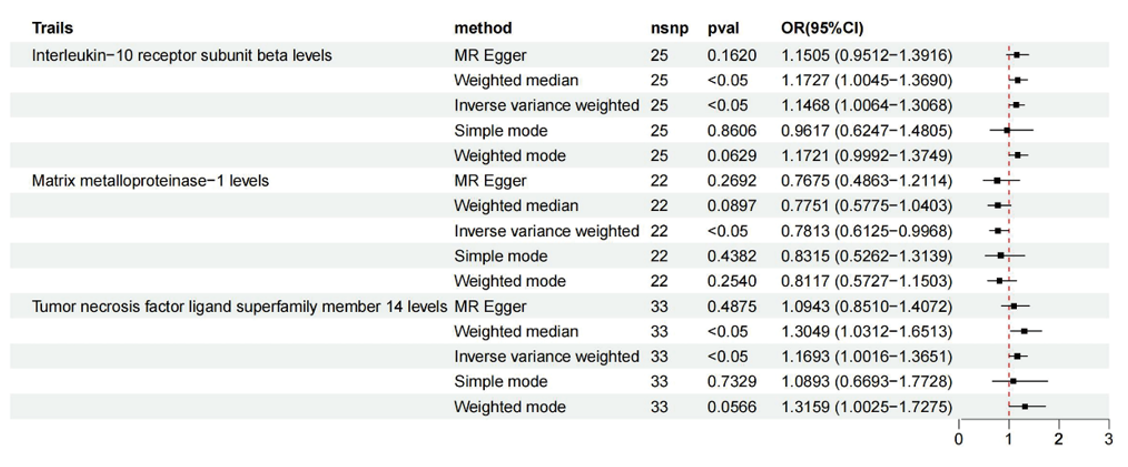
Figure 2 MR results in forward causal effects between inflammatory cytokines and malignant neoplasms of the stomach. snp refers to a single-nucleotide polymorphism; OR represents the odds ratio, and 95% CI indicates the confidence interval.
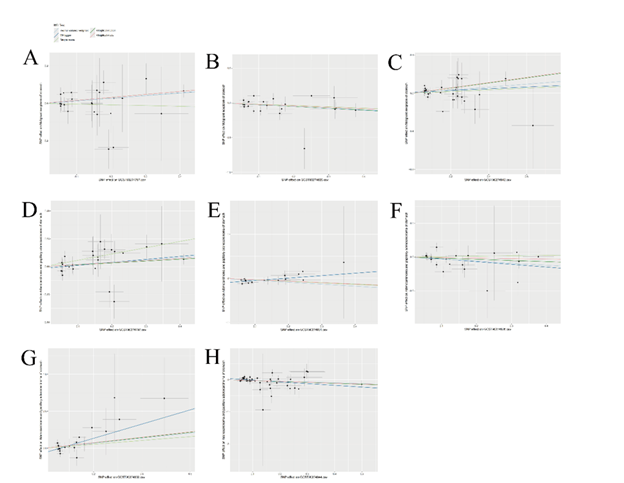
Figure 3 Scatter plots demonstrating causal relationships between exposures (inflammatory cytokines) and outcomes (malignant neoplasm of the stomach and adenocarcinoma and papillary adenocarcinoma of the stomach). (A) Scatter plot of interleukin-10 receptor subunit beta (IL-10RA) levels and malignant neoplasms of the stomach; (B) Scatter plot of matrix metalloproteinase-1 (MMP-1) levels and malignant neoplasms of the stomach; (C) Scatter plot of tumor necrosis factor ligand superfamily member 14 (TNFSF14) levels and malignant neoplasms of the stomach; (D) Scatter plot of interleukin-10 receptor subunit beta levels and adenocarcinoma and papillary adenocarcinoma of the stomach; (E) Scatter plot of interleukin-17A (IL-15RA) levels and adenocarcinoma and papillary adenocarcinoma of the stomach; (F) Scatter plot of matrix metalloproteinase-1 levels and adenocarcinoma and papillary adenocarcinoma of the stomach; (G) Scatter plot of programmed cell death 1 ligand 1 (PD-L1) levels and adenocarcinoma and papillary adenocarcinoma of the stomach; (H) Scatter plot of TNF-related activation-induced cytokine (TRANCE) levels and adenocarcinoma and papillary adenocarcinoma of the stomach.
Forward MR analysis of the associations of 91 inflammatory cytokines with adenocarcinoma and papillary adenocarcinoma of the stomach
The IVW analysis (Figure 4) revealed a causal relationship between the levels of interleukin-10 receptor subunit beta (IL-10RA) and the risk gastric adenocarcinoma (OR = 1.2198; 95% CI: 1.0187-1.4605; P = 0.0307). Additionally, causal associations were observed for several other inflammatory markers: the levels of matrix metalloproteinase-1 (MMP-1) (OR = 0.7813; 95% CI: 0.5520-0.9868; P = 0.0404), interleukin-17A (IL-15RA) levels (OR = 0.6382; 95% CI: 0.4509-0.9033; P = 0.0113), programmed cell death 1 ligand 1 (PD-L1) levels (OR = 1.4040; 95% CI: 1.0197-1.9331; P = 0.0376), and TNF-related activation-induced cytokine (TRANCE) levels (OR = 0.7812; 95% CI: 0.6349-0.9612; P = 0.0196). Notably, the levels of IL-15RA, MMP-1, and TRANCE were associated with a reduced incidence of gastric adenocarcinoma. The Q tests for these five inflammatory cytokines did not reveal any evidence of heterogeneity, and no significant intercepts were found, further supporting the absence of pleiotropy (Supplementary Table S5). Additionally, the 'leave-one-out' analysis demonstrated that no individual SNP significantly influenced the outcomes (see Supplementary Figure S1D-S1H). We present the results of the MR analyses alongside related forest plots (Supplementary Figure S2D-S2H), scatter plots (Figure 3D-3H) and funnel plots (Supplementary Figure S3D-S3H).
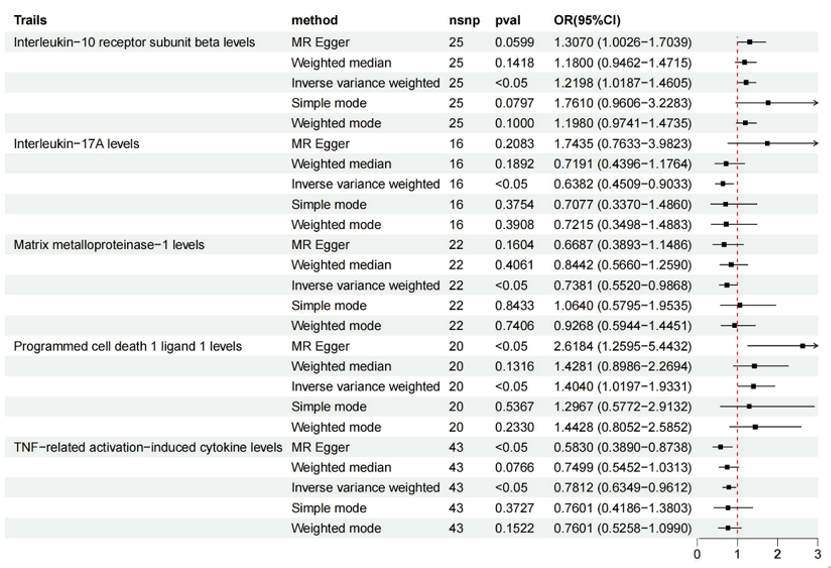
Figure 4 MR results of forward causal effects between inflammatory cytokines and adenocarcinoma and papillary adenocarcinoma of the stomach. snp refers to a single-nucleotide polymorphism; OR represents the odds ratio, and 95% CI indicates the confidence interval.
Reverse MR analysis of inflammatory cytokines
Furthermore, to align with the mediation MR research requirements, we treated the malignant neoplasm of the stomach and adenocarcinoma and papillary adenocarcinoma of the stomach as the exposure and considered the casualty inflammatory cytokines in the above MR analysis as the outcomes in a reverse MR analysis, applying a more stringent significance threshold of P < 1E-05 and utilizing IVW as the main analytical approach. The findings suggested that there was no reverse causation between genetically determined malignant neoplasms of the stomach and adenocarcinoma and elevated levels of inflammatory cytokines, as presented in Figure 5 and Figure 6, along with pleiotropy and heterogeneity tests; the results are detailed in Supplementary Table S6.
This study investigated the causal associations between 91 inflammatory cytokines and stomach malignancies, with a specific focus on adenocarcinoma of the stomach, using the largest available GWAS dataset for MR analysis. The findings revealed that the level of IL-10RA was positively associated with the risk of gastric malignancies and adenocarcinoma, whereas the level of MMP-1 had the opposite relationship with malignant neoplasms of the stomach and with the risk of adenocarcinoma. In addition, the genetic analysis of other cytokines (TNFSF14 and PD-L1) revealed positive evidence to support causality with malignant neoplasms of the stomach and a subgroup of adenocarcinomas, respectively. However, IL-15RA and TRANCE reduced the incidence of gastric adenocarcinoma.
Gastrointestinal cancers rank among the deadliest cancers worldwide, and their associated risk factors share significant similarities. In particular, gastric cancer is strongly linked to chronic H. pylori infection, several lifestyle factors—including alcohol consumption and smoking—and unhealthy dietary habits, such as a high intake of barbecued and processed meats combined with insufficient fruit consumption. Over the past 50 years, the treatment of H. pylori infection and improved food storage practices have contributed to a steady decline in gastric cancer rates.3 Inflammation plays a significant role in the onset and progression of H. pylori infection and gastric cancer.30 Inflammatory cytokines play crucial roles in the tumor microenvironment and are essential components of the innate immune response to danger signals, tissue damage, and infections.31,32 The immune system can affect tumorigenesis in two ways, either facilitating or suppressing tumor growth throughout various phases of cancer progression.33 Our study revealed a causal link between increased levels of IL-10RA in circulation and a greater risk of gastric malignancies, particularly within the adenocarcinoma subgroup. Interleukins play crucial roles in immune and inflammatory responses, significantly affecting the maturation, migration, and adhesion of immune cells.34 IL-10RA is selectively expressed in hematopoietic lineages, including T cells, B cells, NK cells, mast cells, and dendritic cells, and is essential for mediating the biological effects of IL-10.35 The phosphorylation of tyrosine residues on IL-10RA results in critical docking sites that predominantly facilitate the recruitment and activation of the transcription factor STAT3.36 The IL-10/IL-10RA axis is vital for protecting against immune disorders, as demonstrated in conditions involving IL-10 knockout models in vivo and in patients with mutations in IL-10 and its receptors.36–39
MMP1, belonging to a family of zinc-dependent endopeptidases known as matrix metalloproteinases, is an enzyme that plays a critical role in tissue remodeling, wound healing, and extracellular matrix degradation.40 Previous studies have shown that MMP-1 is upregulated in several human cancer tissues and is associated with invasive behavior and tumor progression.41–45 The associated mechanisms include MMP1 gene polymorphisms,42 the PRDX3/ERK signaling pathway,46 which affects human papillomavirus infection, cell-substrate adhesion, focal adhesion, cell-matrix adhesion, proteoglycans, and the PI3K-Akt and MAPK signaling pathways in cancer.44 However, in this study, the levels of MMP1 in the blood negatively affect malignant neoplasms of the stomach and adenocarcinoma, which provides an innovative research direction for MMP1 in the future to illustrate the correlations between blood circulation and histological and immunohistochemical expression in malignant neoplasms of the stomach.
Our study revealed that genetic analysis of additional cytokines, specifically TNFSF14 and PD-L1, provided positive evidence supporting their causal relationships with malignant neoplasms of the stomach and the adenocarcinoma subgroup, respectively. The TNFSF14 protein is expressed primarily on activated T cells, natural killer (NK) cells, and immature dendritic cells (DCs). It functions as both a soluble factor and a membrane-bound type II protein.47 Its expression within tumors significantly influences host immune responses and remodels the tumor microenvironment. TNFSF14 not only helps normalize the tumor vasculature but also stimulates the development of high endothelial venules, which subsequently support the formation of tertiary lymphoid structures. These modifications can improve the efficacy of therapies designed to stimulate antitumor immune responses, such as checkpoint inhibitors and tumor vaccines, thereby enhancing immunotherapeutic approaches for treating gastric cancers.48 PD-L1, on the other hand, is a crucial immune checkpoint protein that interacts with PD-1 on T cells and is key for the T-cell-mediated elimination of cancer cells. However, cancer cells can evade immunity by expressing PD-L1. Specific drugs or combinations of therapies that include PD-L1/PD-1 blockade inhibitors have shown promise in enhancing antitumor immunotherapy for gastric cancer.49,50 Given their roles, TNFSF14 and PD-L1 are potential targets for immunotherapy. Currently, the diagnosis of these biomarkers relies primarily on immunohistochemical methods, but they may also serve as potential serum biomarkers for malignant neoplasms of the stomach.
Our study presents several notable benefits. First, the use of MR design significantly reduces the risk of unmeasured confounding and reverse causation, serving as a primary strength of our research. Second, the bidirectional two-sample MR (TSMR) analysis effectively minimizes bias resulting from confounding factors, environmental influences and reverse causality compared with traditional observational studies. Third, given the multitude of existing, often contentious studies regarding the relationship between inflammatory cytokine levels and malignant neoplasms of the stomach, our findings provide valuable genetic insights that can guide future research in this area. Nonetheless, there are certain limitations to consider.
This study also has several drawbacks. First, our analysis for the MR was conducted exclusively using databases from individuals of European ancestry. As a result, the findings should be cautiously interpreted when considering their applicability to sites, subsets, histological types and stages for malignant neoplasms of the stomach and populations of different races, regions, and ages. Second, while this research identified several inflammatory cytokines associated with an increased risk of malignant neoplasms of the stomach, it is essential to conduct further investigations to clarify their roles in the underlying mechanisms of this disease. Third, our GWAS was limited to a specific range of inflammatory cytokines, analyzing only 91 types, which means that not all relevant cytokines were incorporated into our MR analysis. Future studies should focus on a comprehensive analysis that includes multiple SNPs and gene-gene interactions, as well as the exploration of epigenetics, proteomics, and posttranscriptional modifications to achieve more robust outcomes. Additionally, since the GWAS data were derived from a meta-analysis, we employed inflammatory cytokines with uncorrected P values for our analysis.
An MR study revealed causal relationships between the levels of 91 inflammatory cytokines and malignant neoplasms of the stomach. We confirmed that IL-10RA increased the risk of malignant neoplasms of the stomach and adenocarcinoma subgroup, whereas MMP-1 had the opposite effect on malignant neoplasms of the stomach and adenocarcinoma subgroup. In addition, genetic analysis of TNFSF14 and PD-L1 revealed positive evidence to support causality with malignant neoplasms of the stomach and adenocarcinoma, respectively. However, IL-15RA and TRANCE are protective factors against adenocarcinoma of the stomach. The current study effectively addresses biases related to confounding factors, environmental influences, and reverse causality, enhancing the validity and robustness of our findings. Our results offer reliable genetic support for further investigations into the pathogenic mechanisms by which inflammatory cytokines may be involved in malignant neoplasms of the stomach. Additional research is necessary to evaluate the potential of these cytokines as biomarkers for cancer diagnosis or as targets for therapeutic interventions.
We greatly thank J. H. Zhao for sharing the aggregated 91 inflammatory cytokine GWAS statistical datasets and the participants and investigators of the Finn Gen study. We also sincerely appreciate that all of the consortium research made the summary association statistics data freely accessible.
Hua Wang, Sisi Liu, and Bo Wen performed conceptualization, data acquisition, validation, and writing, as well as providing administrative and funding acquisition. Technical and material support: Shaoyu Xie, Yanping Yang, Sheng Li.
All the information produced or examined throughout this research is contained within this published article and its supplementary materials. Any additional questions can be directed to the corresponding author.
This research was supported by the Hunan Provincial Natural Science Foundation in China (Grant No. 2024JJ9603) and the Science and Technology Program of Shaoyang, Hunan Province (Grant Nos. 2021072ZD,2022GZ4137,2023ZD0104 and 2024PT6164).
The authors declare that they have no competing interests.
Not applicable.
Not applicable.

©2025 Wang, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.