Journal of
eISSN: 2373-633X


Review Article Volume 15 Issue 4
1BSc, MSc Pathophysiology, Department of Clinical Analysis, Hawler Medical University, Iraq
2MBChB, FICMS (Medical), FICMS (Cardiol), Medicine Department, Hawler Medical University, Iraq
3MD, MSc, PhD, Department of Pathology, Hawler Medical University, Iraq
4BSc, MSc, Department of Clinical Pharmacy, Hawler Medical University, Iraq
5MBChB, SHO of Dermatology, Ministry of Health, Iraq
6BSc, MSc, PhD, School of Pharmacy, Nottingham University, UK
Correspondence: Rawaz D Tawfeeq, BSc, MSc Pathophysiology, Department of Clinical Analysis, College of Pharmacy, Hawler Medical University, Erbil, Iraq
Received: August 01, 2024 | Published: August 16, 2024
Citation: Tawfeeq RD, Alwan MH, Ismael AT, et al. Natriuretic peptides as biomarkers and therapeutic target in heart failure. J Cancer Prev Curr Res. 2024;15(4):82-89. DOI: 10.15406/jcpcr.2024.15.00556
Cardiovascular illnesses have the potential to result in the development of heart failure (HF), a fatal phenomenon that can manifest in various forms, making diagnosis and treatment a complex challenge. The current therapeutic approaches for patients with HF are established according to their clinical presentation and echocardiographic measurements. Nevertheless, this method fails to consider the underlying pathophysiological mechanisms involved. Hence, the utilisation of natriuretic peptides (NPs) in conjunction with clinical assessment and echocardiographic outcomes as markers for selecting the optimal treatment for HF, considering the underlying pathophysiology of the condition and current strategies, will contribute to the formulation of future guidelines aimed at enhancing the management of HF. This review study critically analyses the most recent scholarly studies pertaining to natriuretic peptides and their therapeutic implications in the management of both acute and chronic HF. Moreover, we touch up on the clinically available NPs based medication and challenges comes with them in the management of HF. By evaluating the potential of these biomarkers, the study aims to provide a critical analysis of their value in outlining future therapies for HF. The findings are expected to contribute to improving patient outcomes and enhance the quality of provide care for HF patients.
Keywords: heart failure, natriuretic peptide, biomarkers, therapeutic targets, neprilysin inhibitors
HF, heart failure; NP’s, natriuretic peptides; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; CNP, C-type natriuretic peptide; LVEF, left ventricular ejection fraction; RAAS, renin-angiotensin-aldosterone system; AHF, acute heart failure
Heart failure (HF) is a significant health issue, particularly in an aging population. The diagnosis and treatment of HF is challenging and place a burden on the national healthcare system.1 Furthermore, hospitalization of HF patients adds to the cost and affects the quality of life of patients.2 The therapy of chronic ambulatory heart failure has undergone notable progress as a result of the development of pharmacological medicines such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, β-blockers, and mineralocorticoid receptors antagonists. Nevertheless, the incidence of illness and death among individuals diagnosed with HF remains elevated.3 Early selection of evidence-based interventions is vital to prevent morbidities in acute cases of HF.4
The natriuretic peptides (NPs) system consists of three distinct peptides, namely atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and C-type natriuretic peptide (CNP). Furthermore, it includes three separate receptors, specifically natriuretic peptide receptor-A (also known as particulate guanylyl cyclase-A), natriuretic peptide receptor-B (also known as particulate guanylyl cyclase-B), and natriuretic peptide receptor-C (sometimes, the receptor is indicated as the clearance receptor).5 Currently, NPs are widely regarded as the most reliable and widely accepted biomarker for diagnosing acute HF.6 The peptides under consideration serve as indicators of cardiac internal pressure and possess the ability to predict the treatment outcome in the short and long term.7 The European Society of Cardiology has established guidelines that BNP is recognised as a reliable indicator for the purposes of diagnosing, categorising the severity, and predicting the prognosis of HF.8 BNP is a hormone with counter-regulatory properties, playing a role in cardiac remodelling and the regulation of volume homeostasis. Its selection as a useful biomarker in the management of HF is strengthened by the emergence of rapid detection assays.8 This biomarker relies on serial changes in its levels that indicate the progression of HF and facilitate the evaluation of therapeutic interventions.9
The guidelines10,11 emphasise the necessity of employing a therapeutic strategy that takes into account both the left ventricular ejection fraction (LVEF) and the clinical characteristics of the patient.12 Nevertheless, it is important to acknowledge that both methodologies have certain limitations.13–15 Relying solely on the clinical profile as a strategy can occasionally lead to incorrect and oversimplified conclusions, especially in certain contexts. Various pathophysiological pathways may indeed contribute to the development of clinically similar circumstances. However, it is crucial to tailor the treatment approach based on the specific underlying cause.16–18 Regarding this issue, A pragmatic approach grounded in the understanding of pathophysiology and the hemodynamic profile18,19 would be more appropriate, especially when dealing with acute situations. Furthermore, a significant proportion of the proposed and analysed strategies for HF treatment predominantly focus on the management of the chronic stable phase, disregarding the management of acute decompensation episodes. Moreover, an evident deficiency exists in terms of specific instructions for the precise timing and sequence of drug administration, together with the approach to titration, in both acute and chronic situations. Nevertheless, it is important to note that clinical phenotypes may not consistently indicate the exact hemodynamic condition of individuals. Moreover, it should be noted that in the acute clinical environment, relying solely on LVEF assessment may lead to erroneous conclusions. This is mostly due to the fact that LVEF is influenced by fluid volume status and fails to account for the underlying pathophysiological mechanisms contributing to the acute decompensation. In order to address these limitations, a more comprehensive understanding of the underlying physiological processes, coupled with the assessment of hemodynamic factors, can allow the selection of appropriate therapeutic interventions. The primary aim of this current review is to emphasise the potential of NPs in elucidating the therapeutic target of patients with HF. This approach could be employed in combination with the current guidelines of clinical assessment and echocardiographic methodologies to increase the output of the HF management strategy.
The release of NPs is triggered by cardiac strain caused by intravascular fluid overload and HF. In the settings of congestive HF, the process of synthesising and releasing BNP from ventricular cardiomyocytes is triggered due to ventricular hypertrophy and elevated transmural wall stress. BNP and ANP are mostly localised in the cardiac ventricles and atria, respectively. On the other hand, CNP is mostly concentrated in the peripheral vasculature20 (Figure 1).
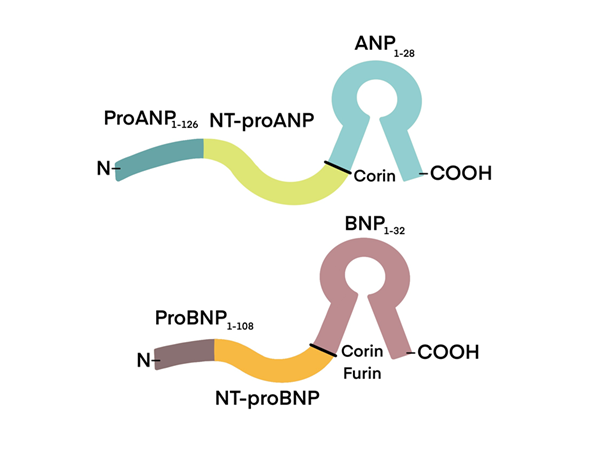
Figure 1 The process of enzymatic breakdown results in to two separate end-products: atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP). ANP is located at the top, while BNP is situated at the bottom. NT-proANP refers to N-terminal pro-atrial natriuretic peptide, while NT-proBNP stands for N-terminal pro-brain natriuretic peptide.
Figure 2 represents the process through which ANP and BNP take their physiological effects. This is achieved by their contact with three distinct natriuretic peptide receptors (NPRs): NPR-A, NPR-B, and NPR-C, with NPR-A being the primary receptor and NPR-C having a comparatively weaker influence. NPR-A exhibits a higher prevalence on blood vessels compared to NPR-B, which is found in relatively lesser quantities. The two receptors are present in adrenal glands and the kidneys as well.21 The process of guanylyl cyclase activation and subsequent signalling via cyclic guanosine monophosphate (cGMP) is facilitated by the binding of ANP and BNP to NPR-A and NPR-B, respectively.22 ANP and, to some degree, BNP hormones undergo degradation through the action of NPR-C upon their interaction to the receptor. Furthermore, neutral endopeptidase serves as an additional mechanism for the inactivation of atrial ANP23 (Figure 2).
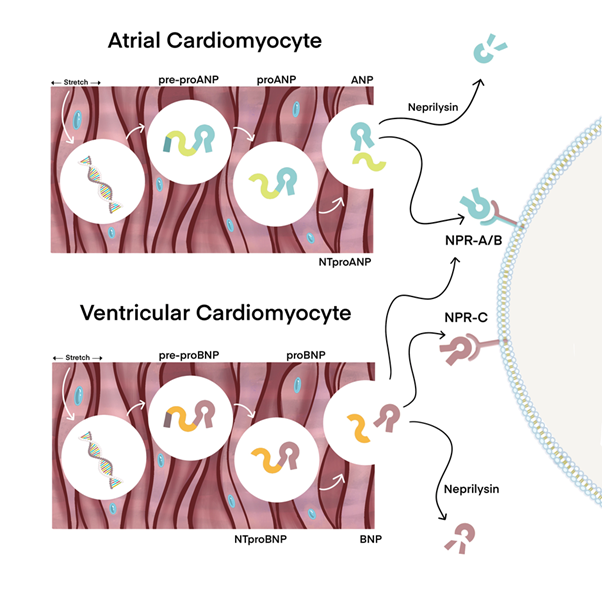
Figure 2 The underlying mechanisms of action of Atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP). ANP and BNP are secreted by the atria and ventricles of the heart, respectively, when the myocardial walls experience expansion.
The impacts of pressure and volume overload are alleviated by the action of circulatory BNP upon its binding to target tissues which leads to an elevation in intracellular cGMP as illustrated in Figure 3. This initiates the commencement of diuresis, vasodilation, natriuresis, and the suppression of the renin-angiotensin-aldosterone system (RAAS).24 In comparison to ANP, BNP is mostly deactivated by neutral endopeptidases present in the bloodstream, and to a smaller degree, through absorption by NPR-C excretion.25 The physiological processes counter-regulate the stimulation of the RAAS and the sympathetic nervous system in HF. Hence, the severity of HF is determined by the level of ANP and BNP (Figure 3).
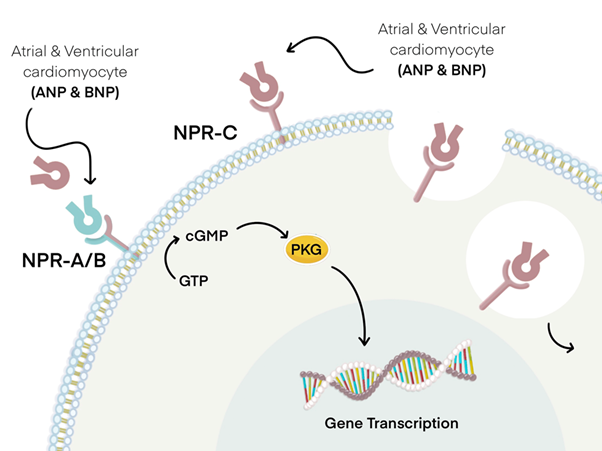
Figure 3 The tissues affected by the action of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP). The occurrence of processes such as diuresis, smooth muscle relaxation and natriuresis is attributed to the contact of both ANP and BNP with natriuretic peptide receptors A (NPR-A) and B (NPR-B). This binding event subsequently leads to the hydrolysis of guanosine triphosphate (GTP) to cyclic guanosine monophosphate (CGMP) with the assistance of cytoplasmic G proteins. This, in turn, triggers a signalling cascade which involves the activation of protein kinase G (PKG) and the transcription of relevant genes, which is contingent upon the specific target tissue. ANP and BNP, albeit to a lower degree, undergo degradation via binding to natriuretic peptide receptor-C (NPR-C), which triggers receptor-mediated endocytosis and subsequent digestion by lysosomes.
The clinical advantages of BNP have gained considerable importance in recent times, despite its first identification in the 1980s. The significance of monitoring serum BNP levels in the identification of acute heart failure (AHF) is underscored by evidence-based practise. According to the current guidelines put forward by the American Heart Association and American College of Cardiology, it is strongly advised, with a class I indication, to measure BNP levels for all hospital admissions related to AHF.11 Cardiac-specific biomarkers, such as BNP, play an important role in distinguishing dyspnea resulting from HF as opposed to pulmonary disorders within the context of emergency department (ED) environments.
The utility of BNP in the identification of HF in ED was first applied by Breathing Not Properly Multinational Study in 2002.26 The research findings indicate that those experiencing dyspnea as a result of AHF exhibited elevated levels of serum BNP in comparison to those with dyspnea stemming from non-cardiac factors. The New York Heart Association (NYHA) categorization indicates that a positive correlation exists between the severity of HF and the proportional raise in serum BNP levels. A diagnostic sensitivity of 90% for the identification of HF could be attained by implementing a 100pg/ml cut-off BNP concentration, with a corresponding specificity of 76%.26 The study conducted in 2005 by PRIDE (Pro-BNP investigation of Acute Dyspnea in the Emergency Department) yielded comparable results.27 This study employed N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels as a diagnostic tool in AHF. NT-proBNP is a peptide hormone that is secreted in an inactive form in conjunction with the active BNP hormone when the heart walls experience stretching or pressure overload. A threshold of 300 pg/mL was utilised to determine the presence of AHF in 600 individual admitted to the ED due to dyspnoea. The diagnostic process exhibited a high level of sensitivity, leading to the identification of AHF cases in the enrolled patient cohort. A total of 600 patients were able to undergo a highly sensitive diagnosis for AHF. The utilisation of NT-proBNP resulted in a diagnostic accuracy of 90% sensitivity and 85% specificity for the detection of HF.27 The remarkable predictive accuracy of BNP and NT-proBNP has been substantiated by several empirical investigations, as evidenced in Table 1.
|
AHF |
Cut-off value |
Sens. |
Spec. |
PPV |
NPV |
Reference |
|
BNP |
<100 pg/ml |
90% |
76% |
79% |
96% |
(26) |
|
NT-proBNP |
<300 pg/ml |
90% |
85% |
76% |
99% |
(27) |
|
MR-proANP |
<120 pmol/l |
97% |
59.9% |
56% |
97.40% |
(28) |
Table 1 The determination of cut off points for the exclusion threshold of natriuretic peptides in cases of acute heart failure
Acute heart failure (AHF) is a pathological state characterised by the sudden onset of impaired cardiac function. The term "sens" refers to sensitivity, which is the ability to detect or perceive stimuli. The concept of specificity refers to the degree to which a statement or description precisely identifies or defines a particular object, event, or phenomenon. The abbreviations PPV and NPV stand for positive predictive values and negative predictive values, respectively. BNP, NT-proBNP and MR-proANP are an abbreviation for B-type natriuretic peptide, N-terminal pro-B-type natriuretic peptide and mid-regional pro-atrial natriuretic peptide, respectively.
The examine on biomarkers in AHF study found that the diagnostic efficacy of mid-regional pro-atrial natriuretic peptide (MR-proANP) was determined to be identical to that of BNP and NT-proBNP within the AHF setting.28 When MR-proANP was utilised with a cut-off value of 120 pmol/L, it exhibited a sensitivity of 97%, a negative predictive value (NPV) of 97.4%, similar to the performance of BNP and NT-proBNP.28 Also, MR-proANP is able to be used in combination with BNP to enhance the performance of the diagnostic test as demonstrated via the C-statistic level which was elevated from 0.787 to 0.816. In addition, the utilisation of MR-proANP proved to be valuable in reaching a conclusive determination regarding the AHF diagnosis in cases where HF individuals' BNP and NT-proBNP levels fell within an indeterminate range or in individuals with a high body mass index, namely those classified as obese.28 The accuracy of these results could be supported by several studies that used MR-proANP for AHF.29, 30
The ease and speed of measuring serum levels of the biomarker BNP transforming it into a potential tool for reducing the duration of hospitalization and the overall cost of treating HF in emergency departments (EDs). A study in EDs to investigate the effect of monitoring NT-proBNP levels on time-to-diagnose (TTD), discharge, and treatment costs for HF patients. The research revealed that measuring NT-proBNP values in EDs could substantially reduce the treatment costs as well as the TTD, and discharge time in patients with HF.31 The Canadian Multicentre Improved Management of Patients With Congestive Heart Failure (IMPROVE-CHF) trial employed the utilisation of NT-proBNP for a total of 534 patients across seven EDs in Canada and reported a reduction in the duration of ED stays and rehospitalization frequency after 60 days by 21% and 45%, respectively, resulting in a decrease in the overall treatment cost.32
Heart failure with preserved ejection fraction (HFpEF), alternatively referred to as diastolic HF, accounts for approximately 50% of the total patients of HF. This condition is more prevalent in patients who possess risk factors like hypertension, diabetes, and obesity.33 The echocardiography is considered the gold standard diagnostic benchmark for HFpEF. It leads to the identification of specified factors associated with diastolic dysfunction in individuals who show classical signs and symptoms of HF. Even though BNP and NT-proBNP levels are not employed for the purpose of differentiating between HFpEF and HFrEF, it is important that HFpEF patient’s exhibit considerably increase of BNP and NT-proBNP values compared to individuals without HF. However, it is crucial to acknowledge that the magnitude of this elevation is comparatively lower in HFpEF in comparision to HFrEF.34 A BNP value of 100 picograms per millilitre (pg/ml) is employed as a diagnostic parameter to identify individuals with symptomatic HFpEF from those without HF. Additionally, the utilisation of a limit of 120 pg/ml of NT-proBNP serves as a definitive feature for the exclusion of HFpEF, as it effectively distinguishes individuals with HFpEF from those without HF. Furthermore, the assessment of NT-proBNP level has the potential to serve as a fundamental prognostic indicator in individuals diagnosed with HFpEF. This is due to its ability to categorise the intensity of the accompanying symptoms, which show a direct link with the NT-proBNP level35 (Figure 4).
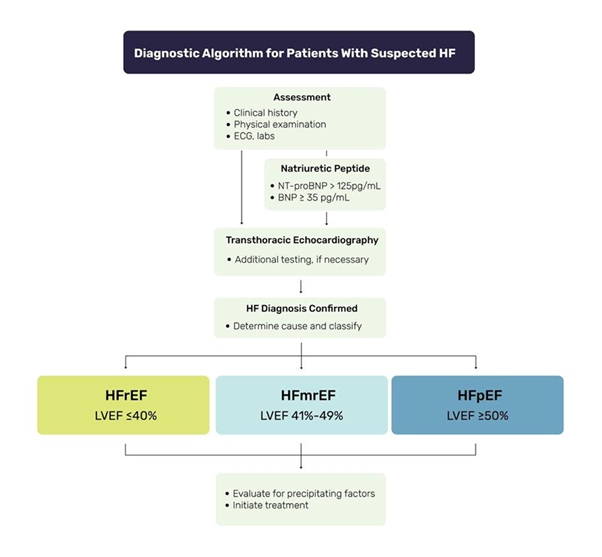
Figure 4 The diagnostic significance of natriuretic peptides in the context of heart failure. The classification of heart failure is determined by the left ventricular ejection fraction (LVEF) as observed in echocardiographic results: it is categorized as heart failure reduced ejection fraction, heart failure mid-range ejection fraction and heart failure preserved ejection fraction when LVEF is ≤40%, 41-49% and ≥50%, respectively.36
AHF patients admitted to hospital who have a BNP blood concentration ranging from 100 to 400 pg/mL, and NT-proBNP concentrations exceeding the specified cutoff values of 300 pg/mL (or the age-adjusted limits for individuals of different age groups are 450, 900, or 1800 pg/mL for individuals younger than 50 years, between 50 and 65 years, and older than 75 years, respectively), are classified as falling within the Grey Zone.36,37 However, interpreting NP levels in this zone poses a challenge in diagnosing acute heart failure since NPs level can be influenced by the presence of certain comorbidities, causing a paradoxical reduction in their levels in patients with AHF and increased values in those without. Although NP levels in the Grey Zone may not be highly accurate in diagnosing AHF, patients with increased NP levels in this zone have worse outcomes, including a higher hazard and reduced mortality rate, than patients with dyspnea but without increased NP values.38 Individuals with NP values inside the Grey Zone may present with preclinical or intermediate heart failure, severe pulmonary conditions, or cardiac ischemia.39 Therefore, the prognostic significance of NP values in the Grey Zone should not be disregarded. Individuals with a prior medical record of HF may have an intermediate elevation of NP levels, particularly after hospitalization due to acute exacerbations of HF in cases where the treatment fails to bring NP levels back to normal. Hence, the interpretation of NP levels should be compared to the patient’s baseline levels when they are symptomatic and euvolemic. An elevation in BNP and NT-proBNP concentrations above the patient's initial levels may suggest the occurrence of an acute worsening of the patient's HF.
Multiple studies have established the utility of BNP levels as a predictive indicator for heart failure. In the year 2002, Berger et al. conducted an evaluation on a sample of 452 ambulatory patients who had a LVEF of less than 35%. The purpose of this study to identify the predictive factors for the incidence of sudden cardiac death (SCD) throughout a three-year period of observation. The study revealed that individuals who exhibited blood BNP levels over 130 pg/ml at the beginning of the study experienced a larger occurrence of SCD. This finding implies that the implementation of implantable cardiac defibrillator therapy should be contemplated for such patients.40 The Angiotensin-Receptor Blocker Valsartan in Chronic Heart Failure (Val-HeFT) study involved a randomized trial of 4300 patients diagnosed with chronic HF. During the course of the trial, baseline serum BNP levels were assessed at two specific intervals: four months and twelve months following enrollment. The study's findings indicate that a substantial decrease in BNP values from the initial measurement is linked to a drop in both morbidity and death rates.41 Hence, the findings derived from these investigations indicate that BNP levels possess significant predictive utility in HF and might provide inclusion in the decision-making framework pertaining to the treatment of HF patients, including the assessment of suitability for implantable cardiac defibrillator therapy.
The 2004 Rapid Emergency Department Heart Failure Outpatients Trial (REDHOT) involved the categorization of 464 dyspneic patients with HF into class II to IV based on the NYHA classification system. Subsequently, an assessment was conducted on the individuals admitted to the ED who had a baseline BNP level over 100 pg/ml. The research revealed that individuals with baseline BNP levels equal to or exceeding 200 pg/ml experienced more unfavourable outcomes throughout a 90-day period, including a higher frequency of HF visits, hospital admissions, and mortality.42 The PRIDE experiment conducted an analysis of baseline NT-proBNP levels in patients who required medical attention at the ED. The study revealed that those with NT-proBNP levels over 986 pg/mL experienced serious HF. Furthermore, it was shown that an individual measurement of NT-proBNP baseline level exceeding the aforementioned threshold served as a robust and exclusive indicator of mortality within the duration of the one-year research period. Following the completion of clinical trials, it was observed that patients diagnosed with both acute and chronic HF saw a 35% rise in the likelihood of mortality for every 100-pg/mL elevation in serum BNP levels.43
A bad prognosis is observed in patients with HF who are hospitalised when stable, non-responsive levels of natriuretic peptides persist while receiving treatment. It is recommended to employ serial measurements of BNP in order to follow patients of this nature.44 The study performed by Cheng et al. (2001) involved a cohort of 72 male veterans who were hospitalised with decompensatory NYHA class III to IV HF. These individuals were subsequently monitored for a period of 30 days following their discharge. The initial BNP levels were assessed during a 24-hour period following admission to the hospital, after which subsequent BNP measures were obtained at regular intervals. Over the course of the trial period, a total of 13 patients suffered death, while 9 patients were readmitted. In both groups, BNP levels increased during hospitalization. However, the remaining patients who showed decreasing BNP levels during hospitalization had less readmissions and higher survival rate.45 In separate research by Bettencourt et al. (2002), serial BNP levels of 50 patients were monitored upon admission for AHF, and BNP levels taken during the patients’ hospitalization were used to predict outcomes after discharge for six months, such as readmission or death. The study revealed a correlation between a reduction in BNP values and an improved prognosis. HF Patients who experienced a decrease in BNP values from an average of 779 ± 608 pg/ml to 643 ± 465 pg/ml during their hospital stay had a lower likelihood of mortality or readmission. Conversely, an increase in BNP levels during hospitalisation was linked toan elevated rate of death or rehospitalization, with a hazard ratio of 3.3 (95% confidence interval, 1.3-8.8).46
The investigation of the possible prognostic usefulness of BNP, NT-proBNP, and MR-proANP has been conducted across diverse clinical databases. The study utilised the Acute Decompensated Heart Failure National Registry (ADHERE) database to evaluate a cohort of 65,275 patients diagnosed with AHF. The findings of this investigation revealed a substantial correlation between elevated BNP values during hospitalisation and in-hospital death.47 In the context of the Get With the Guidelines Heart Failure Registry, a trial was conducted with a total of 99,930 patients diagnosed with AHF. These patients were categorised into sub-groups according to their LVEF and gender. The study findings revealed a notable association between elevated values of BNP and increased mortality rates among AHF patients. This association was detected irrespective of the patients' gender or LVEF status.48 In addition, in the Framingham Offspring sub-study, 3346 asymptomatic patients were evaluated for their serum BNP levels over time in an ambulatory setting, and it was demonstrated that BNP values more than the 80th percentile were linked to cardiovascular events, transient ischemic attack, atrial fibrillation (AF), stroke, HF, or death.49 Furthermore, MR-proANP has also been revealed to indicate prognostic value in mortality of acute and chronic HF. Nadar and Shaikh (2019) reported that in AHF patients, increased mortality up to 4 years from the initial assessment was associated with increasing levels of MR-proANP.6 There is a similar relationship in chronic HF, with increased mortality for more than 5 years after presentation is strongly associated with levels of MR-proANP. According to existing literature, there is a suggestion that the regular monitoring of MR-proANP levels in individuals with chronic HF can enhance the precision of death rate predictions in this patient population.50 To summarise, the research listed above provide evidence of the possible predictive value of BNP, NT-proBNP, and MR-proANP in HF. These outcomes also validate the practise of regularly monitoring these biomarkers to predict mortality.
Patients with aggravated HF, it is recommended that their serum BNP levels be assessed in the ED and compared to their pre-admission medical records. This practise serves to inform and guide the selection of suitable treatment strategies.51 Standardized BNP measurements during hospitalization can aid in monitoring and adjusting treatment for patients. A study conducted by Kazanegra et al., confirmed that regular measurements of BNP and pulmonary capillary wedge pressures in individuals who are hospitalised with acute HF exacerbations helped to identify those patients who were not responding to their current medication.52 Additionally, the available evidence indicates that the implementation of BNP-guided treatment in individuals receiving outpatient care for chronic HF has the potential to enhance patient outcomes. This information can be beneficial to rule out future guidelines for HF treatment.
Recent research has demonstrated signs of the remarkable benefits connected with the utilisation of BNP values as a guiding tool for the management of chronic HF in individuals. The trial conducted in 2009, recorded as BATTLESCARRED, showed similar results related to NT-proBNP-assisted medication for reducing both serial cardiac readmissions and mortality rates.53 The study involved a total of 364 individuals admitted to the hospital due to HF progression. The outcomes of the study showed that therapy guided by NT-proBNP led to a considerably lower mortality rate of 9.1% compared to treatment guided by symptoms during patient monitoring over one year period. A statistically significant difference was recorded in mortality rates with a p-value of 0.03. The STARS-BNP study investigated the effect of plasma BNP-guided therapy on 220 patients classified with class II to III HF of NYHA classification. These patients were already on standard HF therapy, including ACE inhibitors, B-blockers, and diuretics.54 The outcomes of the study demonstrate patients who receive BNP-guided treatment showed a remarkable reduction in mortality due to HF and hospital readmissions compared to those who were therapeutical managed based on clinical and symptomatic improvement guidance. The rates of readmission following a period of fifteen months were 24 per cent in the group who receive BNP based therapy. On the other hand, 52 per cent admission was recorded for the group received clinical and symptomatic based therapy (P<.001). Additionally, the outcomes indicate a decline in the overall mortality rate among individuals receiving NT-proBNP-guided treatment compared to those receiving intensive clinical management and moderate-care interventions throughout the third year of the follow-up. The 2009 TIME-CHF study showed that elderly patients with congestive HF who received NT-proBNP-guided treatment and were between the ages of 60 and 75 experienced higher rates of survival and reduced rehospitalization rates for any cause. These findings were observed during an 18-month follow-up period and were statistically significant with a p-value of less than 0.02.55 However, no improvement was seen in patients older than 75 years. Moreover, a meta-analysis study conducted in 2014 by Troughton and colleagues revealed that BNP-guided treatment led to a decrease in mortality rates due to all causes in HF patients younger than 75 years of age and reduced rehospitalization rates due to HF or other cardiovascular diseases in all age groups with LVEF.56 Overall, this research offers empirical support for the role of BNP-guided treatment in improving results for chronic HF patients.
Despite numerous studies demonstrating the efficacy of NP-guided therapy for HF patients, the potential use of MR-proANP in guided therapy remains underexplored. A study investigating cardiac resynchronization therapy and its association with MR-proANP levels found that patients with decreased serum MR-proANP upon device insertion and six months thereafter were more responsive to treatment than those who did not show a reduction in levels of this biomarker.57
Natriuretic peptides (NPs) have generated an increasing level of interest as therapeutic agents, owing to their vasodilatory, cardiovascular pleiotropic, antifibrotic, renal natriuretic and diuretic effects. The utilisation of human recombinant BNP, known as nesiritide, has been extensively investigated inside medical facilities for the management of AHF in patients. While the intervention demonstrated modest enhancements in dyspnea, it did not exhibit any positive impact on mortality or rates of rehospitalization. Furthermore, it was found to be correlated with elevated occurrences of hypotension.58 Nesiritide did not yield improvements in decongestion or renal function among patients with concomitant renal impairment, nor did it demonstrate favourable outcomes in individuals with chronic heart failure.59 Various delivery routes of BNP, including subcutaneous administration, have been investigated, and have shown improved cardiorenal parameters due to the difficulties associated with nesiritide infusions and side effects as a result of hypotension.60
The alpha-human ANP, known as caperitide, has demonstrated favourable outcomes in individuals diagnosed with AHF and has received regulatory approval in Japan. The administration of caperitide at low doses led to a notable enhancement in the long-term prognosis of AHF.61,62 Caperitide has also been investigated as an initial therapeutic option for the management of HF characterised by preserved blood pressure levels and pulmonary congestion, owing to its demonstrated effectiveness in inducing vasodilation and promoting diuresis. Additionally, Ularitide, an ANP synthesised in the kidneys, has exhibited effectiveness in decreasing filling pressures and enhancing symptoms in individuals diagnosed with AHF.63 The investigational drug Ularitide, with pharmacological properties, is currently in phase III of the TRUE-AHF clinical study.
The year 2015 witnessed an important event in the management of HF with the introduction of the angiotensin-neprilysin inhibitor LCZ696, signifying the advent of a novel era in therapeutic approaches. Neprilysin, which has a degradation effect on a number of peptides including NPs, has been thoroughly investigated. Although it has shown signs of angioedema in prior studies, the pleoitropic effect of NPs was reinforced by the approval of LCZ696 in the context of managing chronic HF. Angiotensin-neprilysin inhibitors were concluded to be more efficacious in the reduction of mortality and rehospitalisation compared to ACE inhibitors alone.64
The PARADIGM-HF study conducted in 2014 revealed compelling evidence of the effect of LCZ696 in obtaining better results of mortality and hospitalisation due to HF. LCZ696 is a novel neprilysin-angiotensin inhibitor that combines the salt form of the both medicine valsartan with sacubitril. LCZ696 study revealed a remarkable improvement in patient outcomes compared to the ACE inhibitor enalapril.64 Neprilysin is the primary hormone that breaks serum BNP. Consequently, neprilysin inhibitors may result in elevation in BNP values, independent of any alterations in the underlying medical condition, like volume overload in AHF. The issue at hand is becoming increasingly concerning due to its potential to disrupt the diagnostic and prognostic properties of BNP. This concern has been observed in conjunction with the widespread clinical application of the Sacubitril-valsartan combination.65
The PARADIGM-HF trial results provided confirmation of the observed elevation in Plasma BNP concentrations among patients administered sacubitril-valsartan compared to those receiving enalapril. However, the combination therapy did not show to have a meaningful effect on the levels of NT-proBNP, since the sacubitril-valsartan group had notably lower NT-proBNP levels.66 However, it is possible that BNP exhibits greater resistance to neprilysin degradation compared to ANP and CNP67 resulting a lesser impact on serum BNP levels by neprilysin inhibitors, as shown by the initial analysis of the PARADIGM-HF trial findings.
Accordingly, certain findings suggest that the measurement of NT-proBNP levels could potentially offer greater reliability in patients who are on sacubitril treatment.68 Nevertheless, further research is necessary to ascertain the precise effect of neprilysin inhibition on BNP. The PARAMOUNT trial investigated the impact of the angiotensin receptor neprilysin inhibitor lCZ686 on patients with maintained ejection fraction and chronic HF. Specifically, it compared the outcomes of patients who received a combination of sacubitril and valsartan with those who received valsartan alone. The levels of NT-proBNP exhibited a considerable decline during the early period, specifically at week 12. Nevertheless, the reduction observed in this study was no longer statistically significant when compared to the control group at week 36. Serum BNP levels were not utilised in the trial.69 Currently, there is a lack of available evidence pertaining to the levels of MR-proANP in patients who are administered a combination of sacubitril and valsartan.
In clinical settings, a range of immunoassays are frequently employed to quantify levels of BNP and NT-proBNP. These immunoassays encompass both rapid and central laboratory assays. In individuals without HF, BNP and NT-proBNP values exhibit similarity, approximately measuring 10 pmol/ml. However, patients diagnosed with HF, NT-proBNP levels tend to have a greater increase compared to BNP levels, potentially reaching up to four times higher than BNP levels. The presence of renal impairment has been identified as a potential factor contributing to the more remarkable increase in NT-proBNP values compared to BNP levels. The technique is designed to quantify the overall concentration of BNP in the bloodstream, encompassing both the physiologically active BNP 1-32 and the inert pro-BNP 1-108. Nevertheless, the measurement of non-biologically active pro-BNP 1-108 is not feasible using this method. The extent of cross-reactivity varies depending on the specific assay type and brand employed, with certain BNP assays exhibiting a cross-reactivity rate of up to 40% with proBNP. The NTproBNP assays demonstrate more specificity, exhibiting minimal cross-reactivity with BNP but significant cross-reactivity with proBNP, reaching levels of up to 200%.70 There exists a requirement for innovative assays capable of discerning between BNP 1-32 and pro-BNP 1-108 isoforms.71
The quantification of the bioactive form of ANP in clinical settings poses difficulties primarily because to its limited duration of biological activity. Consequently, the N-terminal prohormone fragment (NT-proANP) is utilised in clinical practise as an alternative, as it exhibits a longer half-life in serum. Nevertheless, the amounts of NT-proANP do not completely align with the bioactive isoform. Several immunoassays have been devised for the quantification of NT-proANP; however, these assays exhibit unsatisfactory performance as a result of NT-proANP degradation. The central portion of the NT-proANP structure, known as the mid-region (MR-proANP), is the predominant biomarker employed in contemporary clinical studies because to its reduced susceptibility to proteolytic degradation.50 Presently, scientists are directing their attention towards the advancement of assays capable of quantifying fragments of the preprohormone that exhibit stability subsequent to cleavage. However, additional investigation is required to ascertain the therapeutic usefulness of these fragments as an indicator for ANP physiology.
The assessment of BNP and NT-proBNP plays a significant role in the contemporary management of individuals afflicted with HF. The reason being they facilitate the identification of patients who are at an elevated risk of becoming HF, as well as in the diagnosis and prognosis stratification of affected individuals who are already impacted by the condition. Although the potential impact of NPs on directing HF therapy lacks empirical proof, their utility in assessing the efficacy of treatment in acute decompensated HF and chronic HF remains noteworthy. Current evidence shows the potential of NPs in guiding treatments of HF. However, currently, there is a gap in utilizing these as a guideline for the care and treatment of HF. Hence, further investigations are required to enhance comprehension of the utilisation of NPs in guiding HF therapy. With this, NPs could act as a tool to facilitate NP-based treatment future guidelines that is clinically followed.
None.
The authors declare that there are no conflicts of interest.

©2024 Tawfeeq, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.