Journal of
eISSN: 2373-633X


Research Article Volume 16 Issue 3
Research Division, National Ayurveda Research and Training Center (NARTC), Nepal
Correspondence: Amit Man Joshi, National Ayurveda Research and Training Center, National Ayurveda Research and Training Center, Kirtipur, Kathmandu, Bagmati Province, Nepal
Received: June 15, 2025 | Published: June 24, 2025
Citation: Joshi AM, Atri S, Yadav RA, et al. Antiproliferative effects of Paris polyphylla Sm. methanolic extract on A549 human lung cancer cells. J Cancer Prev Curr Res. 2025;16(3):80‒84. DOI: 10.15406/jcpcr.2025.16.00581
Background: Non-small cell lung cancer (NSCLC) accounts for 80% of lung cancer cases, with limited treatment efficacy and high toxicity. Paris polyphylla Sm. (PPS), a Himalayan medicinal plant, contains bioactive steroidal saponins with reported anticancer properties.
Objective: To evaluate the antiproliferative effects of PPS methanolic extract on A549 human lung adenocarcinoma cells.
Methods: PPS rhizomes were collected from Nepal (1741 m altitude), authenticated [National Hebarium and Plant laboratories (KATH) Herbarium], and Soxhlet-extracted with methanol. A549 cells were treated with PPS extract (15.625–250 µg/mL) for 12–48 h. Cell viability was assessed via CCK-8 assay, and IC50 was calculated. Statistical analysis used one-way ANOVA (SPSS v16.0).
Results: PPS extract significantly inhibited A549 proliferation in a dose- and time-dependent manner. Maximum inhibition (42.13% ± 0.33, p<0.01) occurred at 250 µg/mL (48 h), with IC50 of 373.08 µg/mL. Positive control (20 µg/mL fluorouracil) showed 82.27% inhibition.
Conclusion: PPS methanolic extract exhibits potent antiproliferative activity against NSCLC cells, supporting its potential as an adjuvant therapy. Further mechanistic and in vivo studies are warranted.
Keywords: Paris polyphylla Sm, lung cancer, A549 cells, antiproliferation, medicinal plants, natural products, Nepal
Paris polyphylla (P. polyphylla), commonly known as love apple, is a perennial herb with rhizomes that belongs to the Melanthiaceae family. This plant is native to various temperate forests across the Himalayas to Western China, thriving at elevations between 1800 and 3300 meters above sea level.1 It grows best in old-growth forests with over 80% canopy cover, thriving in moist and shady environments.2,3 P. polyphylla is found in several countries, including Bangladesh, Bhutan, China, India, Laos, Myanmar, Nepal, Taiwan, Thailand, and Vietnam.4 In Nepal, it is found at elevations ranging from 1500 to 3500 meters, extending from the west to the east. Locally, it is called 'Satuwa' in Nepali and 'Paris root' in English.5,6
The herb is widely used in ethnobotanical practices in India and Nepal for treating fever, diarrhea, dysentery, gastrointestinal disorders, worm infestations, and as an antidote for poisoning.6,7 In Manipur, the Tangkhul tribes of the Ukhrul district consume raw rhizomes of P. polyphylla to relieve stomach ulcers.8 In Traditional Chinese Medicine (TCM), the rhizome, referred to as Chong Lou, plays a significant role in treating ailments such as abnormal uterine bleeding, cancer, snake bites, and skin diseases.9 It is also a vital ingredient in well-known Chinese medicines like ‘Yunnan Baiyao’ and ‘Gong Xue Ning’ capsules. Moreover, Rhizome Paridis is included in various Chinese patented medicines, such as “Lou lianjiaonang” and “Jinfukangkoufu ye,” which are used as adjunct therapies to support cancer treatment.10 The anticancer properties of P. polyphylla have been documented against various types of cancer, including breast, liver, lung, cervical, gastric, ovarian, and esophageal cancers The anti-cancer activity of Paris polyphylla is primarily attributed to its active constituents—steroidal saponins, particularly polyphyllins (e.g., Polyphyllin I, II, VI, VII). These compounds exert anti-tumor effects by inducing mitochondria-mediated apoptosis, activating caspases (3, 8, and 9), and inhibiting major proliferative signaling pathways such as PI3K/Akt/mTOR and MAPK/ERK. Rather than releasing a specific enzyme, these compounds modulate the expression and activity of several apoptosis-related proteins and enzymes. In our study, the extract was applied directly in in vitro conditions. Traditionally, Paris polyphylla rhizome has been used in oral formulations for internal conditions and topically for inflammation and skin disorders. For systemic anti-cancer applications, oral administration is considered more appropriate, although further pharmacokinetic studies are required.
Besides its anticancer effects, the herb also exhibits antileishmanial, immunostimulant, hemostatic, antihelmintic, and antibacterial activities.18-21 The primary bioactive constituents of this plant are steroidal saponins, particularly Paris saponins, which make up over 80% of its total compounds. These steroidal saponins, such as polyphyllin D, Diosgenin, and Paris saponins I, II, VI, VII, and H, demonstrate anticancer activity comparable to synthetic anticancer drugs.22
Lung cancer is a significant contributor to cancer-related mortality worldwide. Its occurrence and progression are linked to various factors, including oxidative stress, apoptosis, immune system dysfunction, and inflammation. Evidence suggests that the immune response triggered by both endogenous and exogenous toxins plays a crucial role in lung cancer’s pathogenesis.23 Recently, pro-inflammatory cytokines like interleukin (IL)-10 and IL-8 have been implicated in lung cancer development and progression.24 Elevated cytokine levels indicate neutrophilic inflammation in non-small cell lung cancer (NSCLC) patients.25 Furthermore, tumor cells’ production of multiple cytokines influences antitumor immune responses and tumor growth.26
The beneficial effects of herbal medicine in preventing and treating tumors have garnered significant interest. One such medicinal herb is Paris polyphylla Sm. (PPS), which has anti-tumor properties. Phytochemical studies have revealed that its main components, steroidal saponins, exhibit cytotoxicity against various tumor cells, including CCRF leukemia cells, ECA109 esophageal cancer cells, CaEs-17 cells, human promyelocytic leukemia HL-60 cells, human liver carcinoma HepG-2 cells, human gastric cancer BGC-823 cells, human colon adenocarcinoma LoVo cells, and SW-116 cells.17,27-29 Recent findings indicate that steroidal saponins can induce tumor cell apoptosis and inhibit migration in murine lung adenocarcinoma both in vitro and in vivo.30 Additionally, active compounds within steroidal saponins, such as polyphyllin I and polyphyllin D, demonstrate antitumor effects in NSCLC cells.31-33 However, the immunomodulatory and apoptosis-inducing activities of steroidal saponins specifically in lung cancer remain unclear. Thus, the present study aims to evaluate these effects in lung cancer cells, while also exploring potential underlying mechanisms.
Materials
The following materials were utilized in this study: A459 cells were procured from Annapurna Research and Training Center (ARTC, Nepal); Dulbecco’s Modified Eagle Medium (DMEM) and Cell Counting Kit (CCK-8) assay from Sigma-Aldrich; fetal bovine serum (FBS) from Himedia; phosphate-buffered saline (PBS), trypsin, and sodium bicarbonate (NaHCO₃) from Loba Chemie; dimethyl sulfoxide (DMSO) from Merck; penicillin/streptomycin (1x) and glutamine from Gibco; and fluorouracil from Beta Drugs Pvt. Ltd.
Plant material
The rhizomes were collected in December 2023 from Gotikhel, Southern Lalitpur of Bagmati Province, Nepal from an altitude of 1741 m above sea level (latitude: N 27.55, longitude: E 85.33). The plant was identified and authenticated as P. polyphylla Sm. (Melanthiaceae) by National Herbarium and Plant Laboratories (KATH), Godawari, Lalitpur, Nepal. The rhizomes of P. polyphylla Sm. were thoroughly washed and dried in shaded area ground into powder and then passed through a 40 mesh sieve. The powder (100 g) was weighed and extracted with methanol (500 mL) by Soxhlet apparatus for 72 hours. The methanol was recovered by rotary evaporation at 60 °C. A crude extract of 6.21 g was obtained and stored at -20°C until use.
Cell culture
Human lung adenocarcinoma A549 cell line (adherent human alveolar epithelial cell lines) were incubated in DMEM medium, supplemented with 10% FBS, 100 U/mL of penicillin and 100 µg/mL of streptomycin. Cells were maintained in a humidified incubator at 5% CO2 and 37 °C. The medium was renewed every 2 days. These cells were detached by 0.25% trypsin- EDTA and used for seeding. A549 cells were cultured in 25 cm2 flasks with DMEM medium supplemented with 0.1 M L-glutamine, 10% FBS, 100 U/mL of penicillin, 100 µg/mL of streptomycin. Cells were incubated at 37 °C with 5% CO2 in a fully humidified incubator. .
Cell viability assay using CCK-8
The cytotoxic effect of the crude extract was evaluated using the Cell Counting Kit-8 (CCK-8) assay. A total of 100 μL of cell suspension (approximately 5,000 cells per well) was seeded into each well of a 96-well microplate. Two wells containing only 100 μL of culture medium, without cells, were designated as blank wells. The plates were incubated at 37 °C in a humidified atmosphere with 5% CO₂ for 24 hours to allow cell attachment.
After incubation, 10 μL of the test extract at varying concentrations (15.625, 31.25, 62.5, 125, and 250 μg/mL) was added to the respective wells. Two wells containing cells received 10 μL of culture medium instead of the extract and served as control wells. The plates were further incubated for 12, 24, and 48 hours at 37 °C in a 5% CO₂ incubator.
Following the treatment period, 10 μL of CCK-8 solution was added to each well, and the plates were incubated for an additional 4 hours under the same conditions. After incubation, absorbance was measured at 450 nm as the primary wavelength and 630 nm as the reference wavelength using a microplate reader.
The percentage of cell viability was calculated using the following formula:
Viability (%) = [(Abssample− Absblank) / (Abscontrol − Absblank)] × 100.
Where Abssample is the absorbance of wells containing cells, culture medium, CCK-8, and the test extract, Abscontrol is the absorbance of wells containing cells, culture medium, and CCK-8 without the extract and Absblank is the absorbance of wells containing only culture medium (no cells, CCK-8, or extract).
Statistical analysis
All data were presented as means ± standard deviation (SD). Statistical analysis was performed by Statistical Package for the Social Sciences (SPSS) 16.0 software with one-way Analysis of Variance (ANOVA). Significant difference was measured within the groups. The value of p less than 0.05 was considered to be a statistically significant difference.
The results from Table 1. demonstrate the inhibitory effect of different concentrations of PPE (Paris polyphylla extract) on the proliferation of A549 cells after 24 h. The positive control (20 µg/mL) exhibited a significant inhibition of 82.27% ± 0.21, confirming the effectiveness of the reference treatment. Among the PPE-treated groups, the inhibitory effect increased with the concentration of PPE. At the lowest concentration of 15.625 µg/mL, the inhibition was 17.76% ± 0.16. This inhibition effect further increased as the concentration increased, with 22.23% ± 0.26 at 31.25 µg/mL, 26.18% ± 0.23 at 62.5 µg/mL, and 34.15% ± 0.21 at 125 µg/mL. The highest inhibition of 42.13% ± 0.33 was observed at 250 µg/mL of PPE.
|
Group |
Concentration (µg/mL) |
Inhibition (%) |
|
Negative control |
- |
- |
|
Positive control |
20 |
82.27±0.21 |
|
PPE |
15.625 |
17.76±0.16 |
|
PPE |
31.25 |
22.23±0.26 |
|
PPE |
62.5 |
26.18±0.23 |
|
PPE |
125 |
34.15±0.21 |
|
PPE |
250 |
42.13±0.33 |
Table 1 Inhibitory effect of different concentrations of PPE on the proliferation of A549 cells
Statistical analysis revealed that the inhibition at 125 µg/mL was significant with a p-value of less than 0.05, while the inhibition at 250 µg/mL was highly significant with a p-value of less than 0.01, both relative to the control. This suggests that PPE exhibits a dose-dependent inhibitory effect on the proliferation of A549 cells.
The Figure 1. illustrates the inhibitory effect of the methanolic extract of PPS on the proliferation of A549 cells at various concentrations (15.625 to 250 µg/mL) over three different time periods: 12, 24, and 48 hours. The results show a clear dose-dependent and time-dependent inhibition of A549 cell proliferation. As the concentration of the extract increases, the percentage inhibition also increases across all time points. Additionally, longer exposure times lead to greater inhibition at corresponding concentrations. At the lowest concentration of 15.625 µg/mL, the inhibition is around 10% after 12 hours, approximately 17% after 24 hours, and about 20% after 48 hours. At 31.25 µg/mL, inhibition rises to nearly 20% after 12 hours, 22% after 24 hours, and just under 30% after 48 hours. For 62.5 µg/mL, the inhibition is close to 25% after 12 hours, about 26% after 24 hours, and approximately 40% after 48 hours. At 125 µg/mL, the inhibition reaches around 30% after 12 hours, 34% after 24 hours, and 50% after 48 hours. The highest concentration tested, 250 µg/mL, results in about 40% inhibition after 12 hours, 42% after 24 hours, and close to 60% after 48 hours.
Overall, these results indicate that PPE significantly inhibits A549 cell proliferation in a dose-dependent and time-dependent manner, with the greatest inhibition observed at the highest concentration (500 µg/mL) after 48 hours.
The Figure 2. depicts the inhibitory effect of PPE on the proliferation of A549 cells, with the data points fitted to a curve. The concentration of PPE ranges from 0 to 250 µg/mL, and the inhibition percentage is plotted on the y-axis.
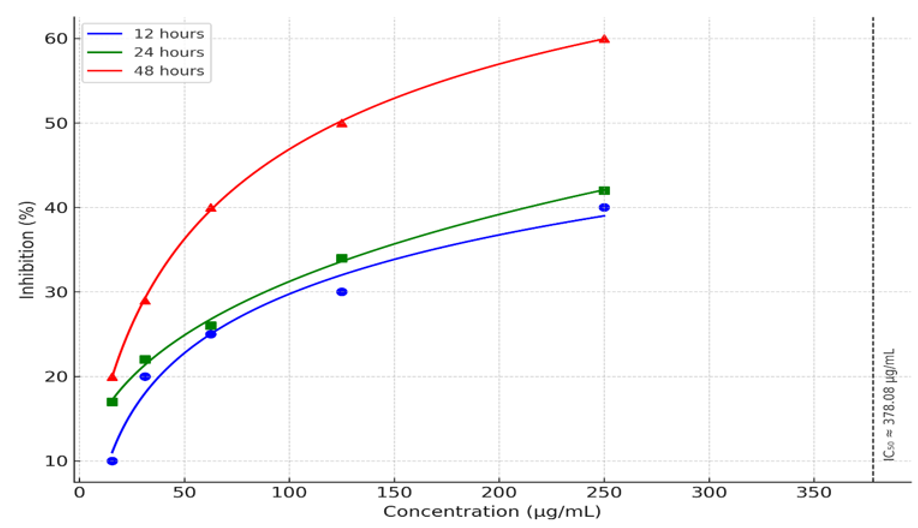
Figure 2 Concentration and time dependent inhibitory effect of methanolic extract of PPE on A549 lung cancer cells.
These results suggest that PPE has a concentration-dependent inhibitory effect on A549 cells, with a moderate level of inhibition at higher concentrations, but it requires a relatively high concentration (373.08 µg/mL) to achieve 50% inhibition. This data supports the potential of PPE as an anti-cancer agent, although further studies at higher concentrations might be necessary to fully characterize its efficacy.
The study showed that higher concentrations of PPE significantly reduce cell viability. This finding is particularly interesting as it suggests a dose-dependent cytotoxic effect of PPE. Our results are consistent with previous research indicating similar outcomes when cells are exposed to high levels of PPE, thereby reinforcing the validity of the present study experimental approach and findings.9,34–36
The reduction in cell viability may be attributed to mechanisms such as apoptosis induction and cell cycle arrest. These processes are crucial for eliminating damaged or unwanted cells and can be triggered by various external stimuli including certain chemical compounds of P. Polyphylla Sm.13,37-47
However, it is important to note that the present study has limitations that must be addressed in future research. Primarily, the study findings are drawn from in vitro experiments which do not fully replicate the complex interactions occurring within living organisms. Therefore, while our findings provide valuable insights into the cytotoxic effects of methanolic extract of P. polyphylla Sm., they must be validated through in vivo studies to ascertain their relevance and applicability in physiological contexts.
Our investigation into the anti-proliferative activity of P. polyphylla Sm. methanolic extract has yielded promising results. Specifically, higher concentrations of the extract exhibit cytotoxic effects, reducing cell viability. These findings suggest that P. polyphylla Sm. extract holds potential as a therapeutic agent for combating cell proliferation. To fully harness this potential, further research is warranted. Mechanistic studies are needed to elucidate the precise pathways through which the extract exerts its cytotoxic effects. Additionally, in vivo validation is crucial to assess its safety and efficacy within living organisms. In summary, the study contributes valuable insights into the pharmacological properties of P. polyphylla Sm. extract, paving the way for future investigations and potential clinical applications.
We acknowledge the laboratory team at NARTC for their assistance in experimental work and data analysis. Appreciation is extended to the Ministry of Health & Population, Government of Nepal, for funding, Annapurna Research Center for providing the A549 cell line, and the National Herbarium and Plant Laboratories (KATH) for authenticating the plant material.
None declared.

©2025 Joshi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.