Journal of
eISSN: 2469 - 2786


Review Article Volume 13 Issue 1
Department of Biotechnology, California State University Channel Islands, USA
Correspondence: Pavan Koti, Department of Biotechnology, California State University Channel Islands One University Drive, Camarillo, CA 93010, USA
Received: April 01, 2025 | Published: April 29, 2025
Citation: Koti P, Bill T. Plant tissue culture and genetic transformation in crop improvement. J Bacteriol Mycol Open Access. 2025;13(1):61-69. DOI: 10.15406/jbmoa.2025.13.00400
Advancements in plant tissue culture and genetic transformation are pivotal in advancing agricultural biotechnology, enhancing crop yields, nutritional value, and environmental resilience. Integration of sophisticated synthetic biology and precise genome editing techniques has significantly refined the accuracy of genetic modifications, enhancing crop yields, nutritional value, and resilience to environmental stresses. The burgeoning role of automation and artificial intelligence revolutionizes tissue culture processes by significantly improving both efficiency and scalability. Key challenges such as public skepticism, regulatory barriers, and technical issues like genetic stability and transformation efficacy are thoroughly discussed. It also explores ethical concerns and diverse global regulatory landscapes, highlighting factors that influence the adoption of these technologies. Case studies confirm the substantial benefits of these biotechnological advances, affirming their potential to dramatically transform agricultural productivity. The paper projects future trends that are likely to further revolutionize this field, emphasizing the necessity for ongoing innovation, ethical vigilance, and unified regulatory frameworks to fully leverage these advancements for global food security.
Keywords: plant tissue culture, genetic transformation, synthetic biology, CRISPR/cas systems, genome editing, regulatory compliance, crop improvement, agricultural biotechnology, ethical considerations, sustainable agriculture
Plant tissue culture and genetic transformation have emerged as cornerstone technologies in agricultural biotechnology, significantly influencing the trajectory of crop improvement.1 Continuous advancements in molecular biology and biotechnology, including the development of new genome editing tools and improved tissue culture techniques, hold promise for overcoming barriers and enhancing the capacity for crop improvement.2
Plant tissue culture and genetic transformation enable the precise manipulation of plant genetics, allowing for the rapid propagation of disease-free plants and the introduction of traits such as higher yield, improved nutrition, and greater stress resilience. As the global population nears 10 billion by 2050 and climatic conditions worsen, these technologies are critical for enhancing crop performance and ensuring food security. However, challenges such as maintaining genetic stability during tissue culture and achieving high transformation efficiency still limit their broader application across diverse crops.2
However, the integration of these technologies into agricultural practices is not without significant challenges. Public skepticism towards genetically modified organisms (GMOs) continues to influence their adoption and regulatory acceptance significantly, often fueled by concerns over potential health and environmental impacts.3
The agricultural biotechnology market plays a critical role in meeting global challenges such as food security, climate resilience, and sustainable farming.4 By focusing on traits like pest resistance and yield improvement, biotechnology is essential for modern agriculture and continues to grow at a substantial rate.5
Global market overview
The agricultural biotechnology market was valued at USD 116 billion in 2023 and is expected to grow to USD 293.35 billion by 2034, with a compound annual growth rate (CAGR) of 8.8%. Biotechnology encompasses a wide range of innovative tools, such as genetic engineering, tissue culture, and marker-assisted breeding, which collectively enable the development of high-yield, pest-resistant, and climate-resilient crops (Figure 1). In the United States, genetically modified (GM) crops like herbicide-resistant soybeans and insect-resistant corn have significantly enhanced agricultural productivity and efficiency. The Asia-Pacific region is also emerging as a major force in agricultural biotechnology, with countries such as China and India investing heavily in research and swiftly adopting biotech solutions to improve food security and agricultural output. China, in particular, has implemented government-backed initiatives to develop pest-resistant cotton and nutrient-enriched rice through extensive funding and supportive policies. Additionally, crop production remains a critical segment in the agricultural biotechnology market, accounting for approximately 30% of total market revenue in 2023, with genetic engineering technologies playing a transformative role in enhancing both crop yield and quality. As these technologies continue to meet critical agricultural needs, the global agricultural biotechnology sector is expected to gain even broader acceptance.4
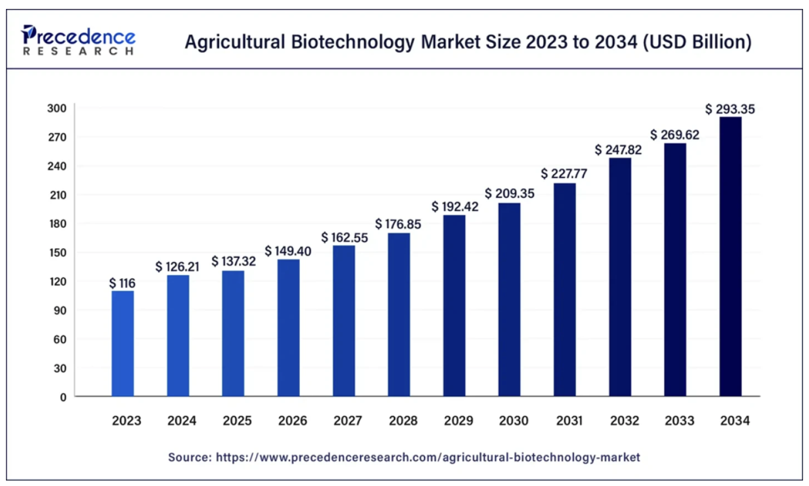
Figure 1 Agricultural biotechnology market growth (2023-2034). This bar chart shows the agricultural biotechnology market expanding from USD 116 billion in 2023 to USD 293.35 billion by 2034, with a steady CAGR of 8.8%. The increase reflects advances in genetic engineering and other biotechnologies that improve crop performance and address food security.
A major driver of this market expansion is the rising demand for sustainable and efficient farming practices that address challenges like food insecurity and environmental degradation. In North America, the agricultural biotechnology market is projected to maintain strong growth as precision breeding and genome editing technologies are increasingly introduced, aligning well with a regulatory environment that supports innovation.5 Moreover, in Asia-Pacific countries like China and India, marker-assisted breeding and tissue culture methods are being widely adopted to develop disease-resistant and drought-tolerant crops, helping these nations to meet the agricultural demands of large and growing populations.5
North America continues to lead the global agricultural biotechnology market, buoyed by robust regulatory frameworks, strong research funding, and widespread adoption of genetically modified crops. Additionally, innovations in agricultural biotechnology are contributing significantly to environmental sustainability by reducing reliance on chemical inputs such as pesticides and fertilizers.6
The principle of totipotency, the ability of a single plant cell to regenerate into a whole plant, is the foundation of plant tissue culture.7 Key to this process is the regulation of growth hormones, particularly auxins, which promote root formation, and cytokinins, which encourage shoot development.8 The balance between these hormones determines the regenerative pathway, making them essential for tissue culture success.7
A sterile environment is also crucial to prevent contamination, and the culture medium must be optimized with essential nutrients such as nitrogen, potassium, and key micronutrients like iron and zinc for proper plant cell growth and differentiation. Recent advancements have improved the precision of hormone regulation and nutrient optimization, enhancing the efficiency of plant tissue culture methods.8
Callus culture
Callus culture is a crucial technique in plant tissue culture, allowing for the formation of undifferentiated cell masses from plant explants through the manipulation of plant growth regulators. The balance between auxins, such as 1-naphthaleneacetic acid (NAA), and cytokinins, such as 6-benzylaminopurine (6-BA), is essential for inducing callus formation and regenerating shoots, as demonstrated in white clover (Trifolium repens). This hormone balance controls the developmental pathway of the callus, either leading to root or shoot formation depending on the relative concentrations of these regulators.9
In addition to its role in regeneration, callus culture plays an essential part in genetic transformation, where callus serves as a platform for introducing foreign genes into plants through methods such as Agrobacterium-mediated transformation. For example, in tobacco (Nicotiana tabacum), the overexpression of transcription factors like LEC2 has been shown to significantly enhance callus formation and shoot regeneration without the need for exogenous hormones (Figure 2).10
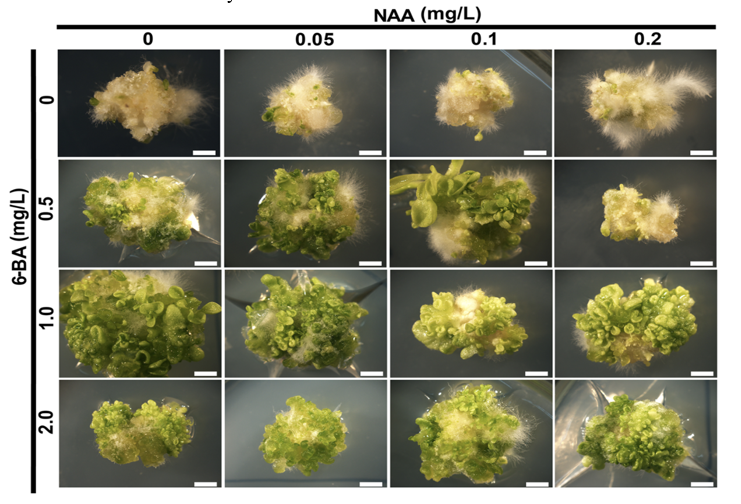
Figure 2 Callus induction and shoot regeneration in tobacco plants under varying concentrations of NAA and 6-BA.
The figure illustrates the process of callus induction and shoot regeneration in tobacco, demonstrating how different concentrations of NAA and 6-BA influence shoot formation from embryogenic callus. This technique highlights the importance of plant growth regulators in facilitating crop improvement through the regeneration of genetically modified plants
Somatic embryogenesis
Somatic embryogenesis (SE) is a critical process in plant tissue culture, enabling the regeneration of entire plants from somatic cells rather than through traditional sexual reproduction pathways.9-12 This process involves inducing somatic cells to become totipotent, allowing them to differentiate into any cell type and ultimately form a complete plant.12 SE has significant applications in agriculture, particularly for the propagation of genetically identical plants at a large scale, which is especially valuable for crops that are difficult to reproduce through conventional methods, such as soybeans and bamboo.13
The success of SE is influenced by several factors, including the plant’s genotype, the type of explant used, and the composition of the growth medium.11 For instance, in soybean cultivars like 'Snowy', SE induction from immature cotyledons has been optimized, resulting in high regeneration rates and making them suitable candidates for genetic transformation.12 Plant growth regulators, particularly auxins such as 2,4-Dichlorophenoxyacetic acid (2,4-D), play a pivotal role in promoting callus formation and embryo initiation during SE.9 Additionally, transcription factors like WUSCHEL-RELATED HOMEOBOX (WOX) are crucial for maintaining cellular totipotency and regulating the developmental pathways of somatic embryos.11
Organogenesis
Organogenesis is a fundamental process in plant tissue culture that facilitates the regeneration of specific organs, such as shoots and roots, from explants by utilizing the totipotency of plant cells. This regeneration can occur through direct organogenesis, where organs form directly from the explant, or indirect organogenesis, which involves the intermediate formation of a pluripotent callus that later differentiates into organs. Both pathways are crucial for plant regeneration and for recovering viable plantlets from tissue cultures.14
Studies on Corydalis saxicola have demonstrated that the application of specific plant growth regulators, such as benzyladenine (BA) and thidiazuron (TDZ), is essential for inducing callus formation and promoting adventitious shoot regeneration. Explants cultured on Murashige and Skoog (MS) medium responded positively to varying concentrations of these hormones, emphasizing the importance of medium composition and hormonal balance in successful organogenesis. Additionally, callus induction and subsequent organ formation were further influenced by factors like medium pH and the concentration of other growth regulators such as naphthaleneacetic acid (NAA) and BA (Figure 3).15
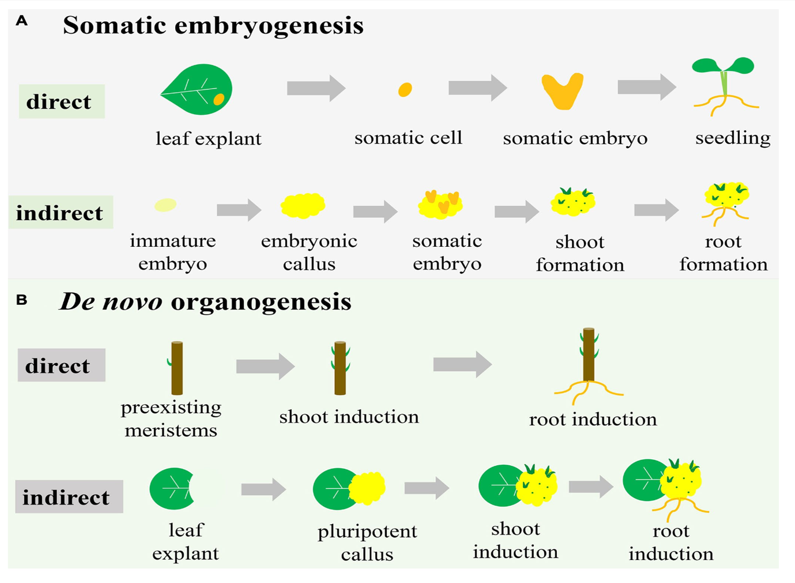
Figure 3 Pathways of somatic embryogenesis and de novo organogenesis in plant regeneration.
Somatic embryogenesis follows direct and indirect pathways from leaf explants and immature embryos, leading to shoot and root formation. (B) De novo organogenesis demonstrates shoot and root induction from preexisting meristems via direct pathways or pluripotent callus formation in indirect pathways.
Micropropagation
Micropropagation is a highly efficient technique used for the large-scale production of genetically identical, disease-free plants in controlled environments. This technique involves four key stages: initiation, where sterilized explants are introduced into a nutrient medium; multiplication, where shoot formation is promoted using cytokinins like benzyladenine (BA); rooting, induced by auxins such as indole-3-butyric acid (IBA); and acclimatization, where plantlets are transitioned from in vitro conditions to soil environments.16
Recent advancements in bioreactor systems have further enhanced the scalability of micropropagation by enabling mass propagation in liquid cultures, significantly increasing the production capacity of commercial micropropagation facilities.16 These innovations have made micropropagation an indispensable tool for large-scale agricultural and forestry operations. Furthermore, the ability to produce uniform plants continuously, regardless of seasonal constraints, has solidified micropropagation as a cornerstone of modern plant biotechnology.17
Genetic transformation techniques in plants are essential for introducing new traits and enhancing crop resilience, employing methods like Agrobacterium-mediated transformation, biolistic particle delivery, and CRISPR/Cas9 genome editing, each tailored to specific applications and species requirements.18-21
Agrobacterium-mediated transformation
Agrobacterium-mediated transformation is a cornerstone in plant biotechnology, extensively utilized for genetic modification of a wide range of plant species, particularly dicots. This method leverages the natural mechanism of Agrobacterium tumefaciens, which transfers a part of its DNA known as T-DNA to the plant cell. This T-DNA is integrated into the plant genome, allowing for the stable expression of new genes. Over the years, the understanding of the interaction between Agrobacterium and plant cells has significantly deepened, revealing the complex molecular events that underpin this natural genetic transformation process.18
The efficiency of Agrobacterium-mediated transformation varies among different plant species, influenced by factors such as the plant's natural susceptibility to Agrobacterium and the specific conditions used during the transformation process. For instance, the transformation of Arabidopsis and tomato using this method is highly efficient due to their natural receptiveness to Agrobacterium infection. However, in monocots, which are generally less susceptible, modifications to the method or use of specific strains of Agrobacterium are required to achieve successful transformation (Figure 4).18
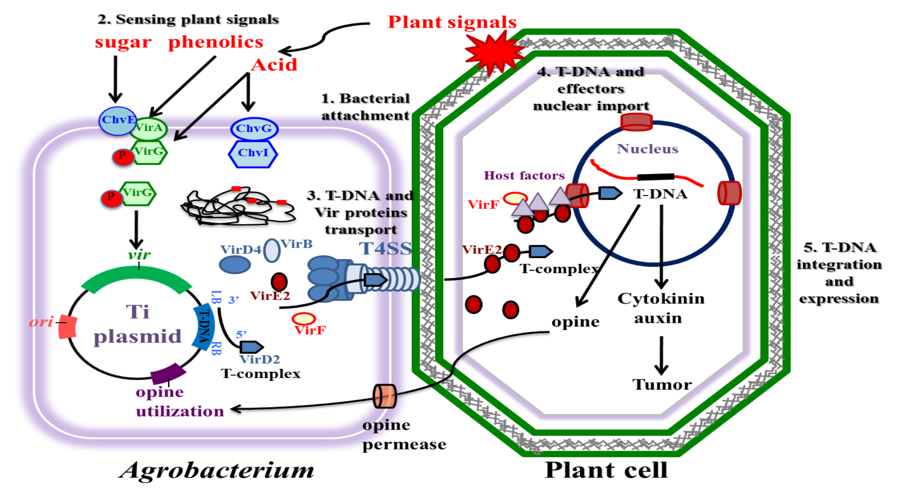
Figure 4 Major steps of the Agrobacterium tumefaciens-mediated plant transformation process.
The process involves bacterial attachment, sensing of plant signals, transduction of T-DNA and virulence proteins, nuclear import of T-DNA into plant cells, and integration into the plant genome. Opine utilization and effector proteins are key elements of the transformation process.
Biolistic particle deliver
Biolistic particle delivery, or the gene gun method, is another powerful technique used to introduce genetic material into plant cells. This method involves propelling microscopic particles coated with DNA into plant tissues at high velocity. It is particularly useful for transforming plant species that are recalcitrant to Agrobacterium-mediated transformation, such as many monocots including corn and wheat. The biolistic method allows DNA to bypass the cell wall and membrane barriers, directly entering the cell where it can integrate into the plant genome.19
Despite its versatility, the biolistic method can sometimes lead to random integration of the transgene, which may result in variable gene expression levels among transformed plants. Moreover, this technique often results in the insertion of multiple copies of the transgene, which can complicate genetic analysis and potentially lead to gene silencing issues. The physical nature of this transformation process also requires specialized equipment and can cause significant damage to the target cells, which may affect regeneration and development of the transformed plants (Figure 5).19
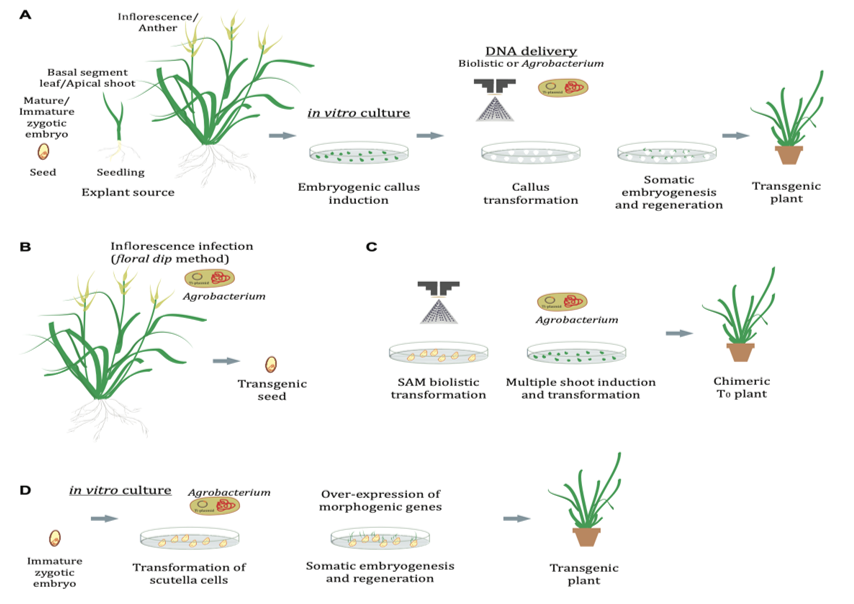
Figure 5 Genetic transformation in grasses.
Transformation of calli and regeneration by indirect somatic embryogenesis, with explants sourced from basal segments and zygotic embryos. (B) Agrobacterium infection via floral dip method. (C) SAM biolistic transformation leading to multiple shoot induction. (D) Transformation of scutella cells via somatic embryogenesis.
CRISPR/Cas9 genome editing
CRISPR/Cas9 genome editing represents a revolutionary advance in plant genetic transformation, offering unparalleled precision in making targeted genetic modifications. Utilizing a simple guide RNA to direct the Cas9 nuclease to specific genomic locations, this method allows for precise cuts and edits within the plant genome, enabling the deletion, insertion, or modification of plant genes. This technology has been rapidly adopted across various plant species due to its efficiency and simplicity compared to earlier genetic engineering techniques.20,21
CRISPR/Cas9 not only enhances the toolkit for basic plant science research but also holds promise for rapidly introducing traits such as disease resistance, drought tolerance, and enhanced nutritional content in crops. However, the technique does face challenges such as potential off-target effects and regulatory hurdles for genetically edited crops. Despite these challenges, CRISPR/Cas9 continues to evolve, with new variants being developed that expand its potential applications and improve specificity and efficiency.20,21
Plant tissue culture and genetic transformation have markedly advanced agricultural practices by enhancing crucial crop traits such as disease resistance, drought and stress tolerance, and nutritional content. These techniques exemplify the power of biotechnology in meeting global food security challenges by enabling precise genetic.22-24
Disease resistance
Disease resistance engineered through genetic transformation has significantly decreased the need for chemical pesticides.22 For instance, Bt cotton, genetically modified to produce Bacillus thuringiensis (Bt) toxins, has shown remarkable effectiveness against major pests, substantially reducing pesticide use and environmental impact. This transformation not only enhances crop health and yield but also supports sustainable agricultural practices. This biotechnological advancement contributes significantly to integrated pest management strategies globally (Figure 6).22,23
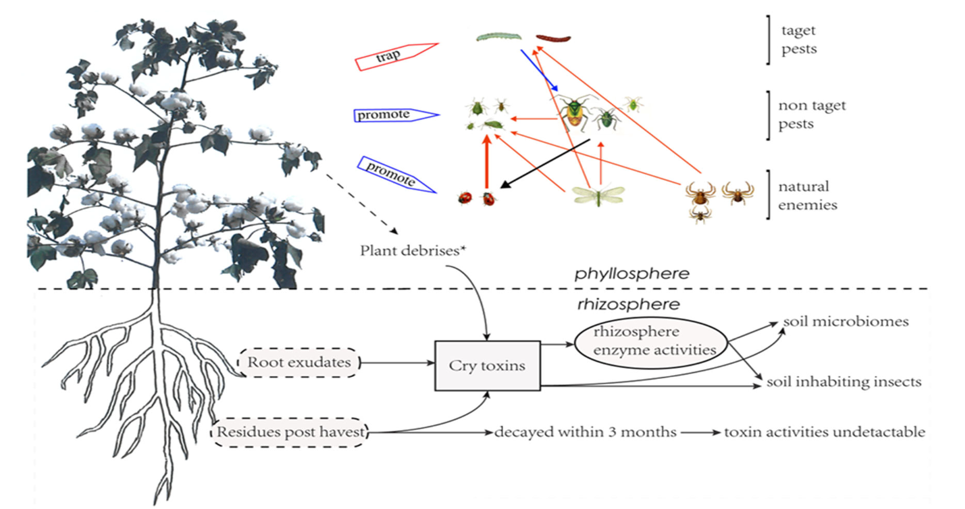
Figure 6 Changes in pest-community interactions due to Bt cotton and Bt toxins.
The diagram illustrates the interaction between Bt cotton, soil, and pest species. Cry toxins produced by Bt cotton affect root exudates, soil microbiomes, and pest species. Residual toxins persist in soil for up to 3 months, affecting both target and non-target pest species, while promoting beneficial natural enemies.
Drought and stress tolerance
Advances in genetic engineering have also facilitated the development of crops with improved drought and stress tolerance, which are essential for adapting agriculture to climate variability.24.25 Through techniques like CRISPR/Cas9, genes linked to water retention and stress response are targeted, resulting in crops that can thrive under suboptimal water conditions.24 This is particularly vital in arid regions where water scarcity poses a significant threat to crop production, thereby ensuring consistent yields and reducing the risk of food shortages.25
Nutritional enhancement and future directions in agricultural biotechnology
Nutritional enhancement via genetic engineering is vividly illustrated by Golden Rice, which has been modified to produce beta-carotene in its grains. This innovation addresses vitamin A deficiency, a serious health issue in many parts of the world where rice is a dietary staple. The introduction of Golden Rice serves as a strategic intervention aimed at alleviating micronutrient malnutrition through dietary improvements, offering a sustainable alternative to supplements and dietary diversification.24,25
Beyond Golden Rice, the integration of plant tissue culture and genetic transformation technologies in agriculture has facilitated the development of crops with enhanced qualities, such as increased nutritional value, improved texture, and greater environmental sustainability. Future advancements are expected to further harness these technologies, focusing on increasing crop efficiency, adapting to changing environmental conditions, and enhancing food quality and safety. These innovations are pivotal in transforming agricultural practices and ensuring that food systems can sustainably meet the needs of a growing global population.26,27
Since their commercial debut in the mid-1990s, genetically modified (GM) crops have transformed agriculture by boosting yields, reducing pesticide use, and enhancing farm incomes worldwide.28-30 These advancements have contributed to economic and environmental sustainability while reshaping socio-economic dynamics within agricultural communities.21,31,32
Successful applications of genetic engineering
Advances in citrus genetic engineering have demonstrated significant success in combating various diseases such as citrus canker and huanglongbing (HLB), also known as citrus greening disease. Genetic engineering methods, particularly CRISPR-based genome editing, have proven effective in developing disease-resistant citrus varieties, exhibiting a high degree of precision and effectiveness. Transgenic citrus plants showing resistance to these diseases are crucial due to the high economic impact and the challenges in managing them through conventional methods. By reducing yield losses and minimizing the reliance on expensive chemical treatments, these genetically engineered citrus varieties have significantly contributed to safeguarding farmer incomes and stabilizing citrus production in affected regions.28
This genetic approach has also been particularly effective in crops where traditional breeding methods are less feasible. Transgenic squash and papaya resistant to viral diseases have been commercially successful in the United States for over two decades, showcasing the practical application and long-term viability of genetic engineering techniques. The commercialization of virus-resistant papaya notably saved the Hawaiian papaya industry from economic collapse, preserving farmer livelihoods and sustaining the regional agricultural economy.29
Environmental benefits of GM crops
Genetically modified (GM) crops have significantly reduced environmental impacts through decreased pesticide use and reduced carbon emissions.30,31 The introduction of GM insect-resistant and herbicide-tolerant crops has led to a notable reduction in pesticide usage by 618.7 million kilograms, marking an 8.1% decrease. This reduction has also improved the Environmental Impact Quotient (EIQ) by 18.6%, reflecting a decreased ecological footprint from agricultural practices.30
Additionally, GM crops have contributed to significant reductions in carbon emissions. Enabled by reduced tillage practices, which lower fuel consumption and enhance soil carbon sequestration, the impact in 2015 alone was equivalent to removing 11.9 million cars from the roads, underscoring the role of GM crops in combating climate change.32 These advances promote sustainable farming by minimizing reliance on chemical inputs, enhancing biodiversity, and conserving soil health, thus supporting more resilient agricultural ecosystems.31,32
Socio-cultural impacts of GM crop adoption
GM crops significantly impact socio-economics beyond direct financial gains. They influence educational opportunities, knowledge-sharing practices, and broader social learning within farming communities. The adoption of GM technology alters traditional agricultural systems, particularly affecting seed-sharing practices and causing shifts in cultural norms and social relations.33
The necessity for farmers to purchase new seeds each season, often due to intellectual property protections, disrupts traditional customs surrounding seed saving and exchange, leading to changes in community cohesion and farming independence. Additionally, shifts in labor distribution brought by new agricultural practices influence gender roles and reshape community decision-making regarding technology adoption. These socio-cultural and economic shifts prompt broader debates on ethical values, social equity, and the equitable distribution of biotechnology benefits within rural communities.33
Challenges and limitations in genetic transformation
Genetic transformation using advanced technologies like CRISPR/Cas faces significant challenges. Technical issues such as off-target effects and variable efficiencies across species complicate applications.34,35 Biological and ecological risks include gene flow and potential ecological disruptions.36 Additionally, scalability and reproducibility are constrained by technical complexities and regulatory frameworks, influencing the consistency of outcomes across diverse environments.37-39
Technical challenges
Gene editing tools such as CRISPR/Cas, TALENs, and ZFNs have markedly advanced genetic engineering in agriculture, yet they encounter significant technical hurdles that limit their application. These challenges primarily involve variability in editing efficiency, which is often low and inconsistent across different species due to complex tool assembly and the risk of unintended off-target mutations. For example, ZFNs generally show an editing efficiency of only 1% to 10%, compounded by their potential to induce non-specific genetic changes. TALENs, though more specific, are hindered by their large size which complicates cellular delivery and expression (Figure 7).34 The CRISPR/Cas system, despite its relative simplicity and higher efficiency, also struggles with issues like stringent protospacer adjacent motif (PAM) requirements and off-target effects, which can lead to undesirable genetic alterations. Additionally, the delivery mechanisms for these tools, such as Agrobacterium-mediated transformation or biolistics, often lead to random DNA integration or can damage the plant tissue, posing further challenges to precise and safe genetic modifications.35
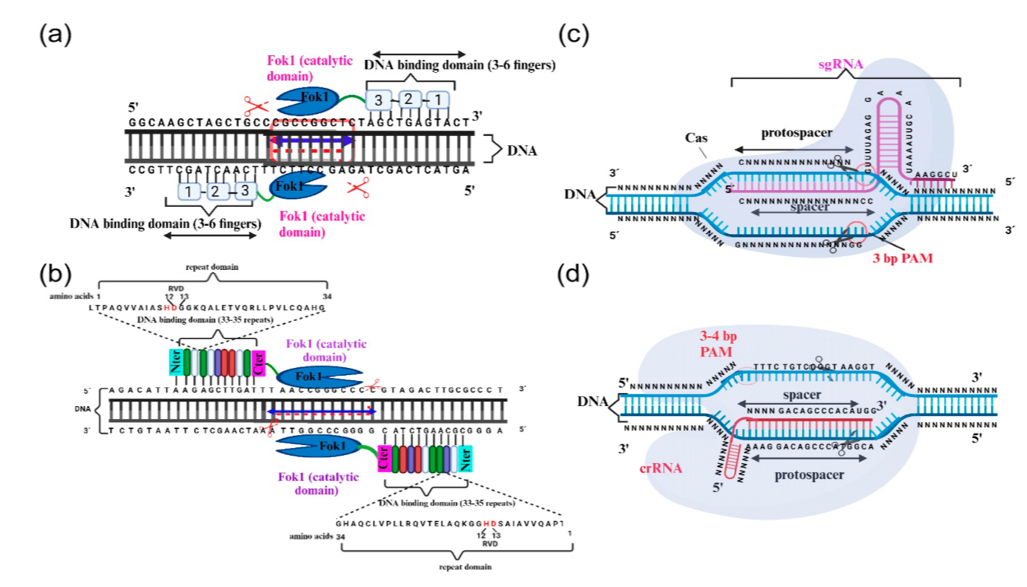
Figure 7 Overview of gene-editing systems: ZFNs, TALENs, CRISPR/Cas9, and CRISPR/Cas12a
ZFNs use zinc finger proteins to target specific DNA sequences and employ FokI endonuclease domains to create double-strand breaks. (b) TALENs function similarly but utilize TALE domains for DNA sequence recognition, activating FokI to cleave the DNA. (c) CRISPR/Cas9 employs a guide RNA to direct the Cas9 enzyme to a DNA site next to a protospacer adjacent motif (PAM) for precise cutting. (d) CRISPR/Cas12a uses a CRISPR RNA (crRNA) to guide Cas12a to the DNA, requiring a PAM sequence for effective DNA cleavage. Each panel highlights the targeted DNA interaction and cleavage mechanism specific to each system.
Biological and ecological challenges
Genetically modified (GM) crops present significant biological and ecological challenges, particularly due to gene flow which can lead to unintended spread of transgenic traits to wild or weedy relatives, increasing their invasiveness and disrupting local ecosystems.36 This gene flow can reduce biodiversity by altering competition dynamics and displacing native species, while herbicide-resistant crops have led to the evolution of resistant weeds that necessitate higher herbicide use, impacting non-target species and reducing agricultural biodiversity.37 Furthermore, the persistence of GM traits in the environment can lead to genetic homogenization, diminishing the genetic diversity and resilience of natural ecosystems, making them less adaptable to environmental changes and pest pressures. These ecological impacts necessitate careful management and monitoring of GM crops to mitigate their potential negative effects on biodiversity and ecosystem health.38
Scalability and reproducibility
Scalability and reproducibility are crucial challenges in the genetic transformation of crops using CRISPR/Cas systems, especially in the context of cereal crop improvement.39 The CRISPR technology, while transformative, encounters significant hurdles when scaled for agricultural applications across diverse environment. These challenges stem from the variability in gene-editing outcomes influenced by environmental factors and the genetic diversity among crop species, which often result in inconsistent phenotypic expressions. Additionally, technical issues such as off-target effects and the specificity required in the protospacer adjacent motif (PAM) sequences limit the predictability and uniformity of the desired genetic modifications.40 Furthermore, the scalability of CRISPR applications is constrained by the extensive regulatory frameworks governing genetically modified organisms, which vary significantly across regions and impact public acceptance and adoption rates.39,40 Effective implementation on a larger scale requires overcoming these reproducibility and regulatory challenges to ensure that genetically edited crops perform reliably in different agricultural settings.40,41
The ethical and regulatory landscape of biotechnology, particularly for GMOs, encompasses debates over ecological risks, the ethics of altering natural organisms, and balancing these concerns with benefits like food security.42,43 Regulatory approaches differ globally; the EU enforces strict regulations, while the U.S. applies a more permissive, product-focused policy.44,45 Public perception, influenced by media and values on food safety, shapes the discourse, highlighting the need for transparent, science-based policies to address evolving concerns.46,47
Ethical implications of GMOs
The ethical implications of genetically modified organisms (GMOs) raise significant concerns and debates, particularly regarding their potential impact on human health, environmental risks, and socioeconomic effects.41 Critics argue that GMOs may harm biodiversity and disrupt ecosystems, as highlighted by studies suggesting that GM crops could indirectly harm non-target species and reduce genetic diversity within agricultural systems.42 On the other hand, proponents advocate for the benefits of GMOs in enhancing food security and agricultural efficiency, especially in regions plagued by food scarcity and harsh farming conditions. Ethical discussions also focus on the monopolization of seed supply by a few large corporations, which could undermine traditional farming practices and threaten food sovereignty. Such concerns necessitate rigorous ethical reviews and transparent, inclusive decision-making processes to ensure that GMO deployment aligns with broader societal values and contributes positively to sustainable development.43
Regulatory landscape
The regulatory frameworks governing genetically modified (GM) crops display significant global diversity, reflecting varying national priorities in biosafety, public health, and environmental protection. In the European Union, regulations are notably stringent, with a process-based system that mandates comprehensive assessments of GM crops before they can be approved. This method contrasts sharply with the United States' product-based approach, which focuses primarily on the characteristics of the final product rather than the genetic modification processes involved.44 In emerging economies, the adoption of GM technologies is often promoted as a strategy to boost agricultural productivity and enhance food security, leading to more streamlined regulatory procedures.45 Meanwhile, countries like Brazil and Argentina have crafted regulatory frameworks that facilitate the rapid adoption of new biotechnological innovations, including genome editing, effectively combining both process- and product-based regulatory elements.46
Public perception and policy response
Public perception of genetically modified organisms (GMOs) varies widely and is strongly shaped by media portrayals, which often emphasize potential risks over benefits, leading to considerable skepticism and misinformation among consumers. This skepticism is heightened by sensationalized media coverage of preliminary studies that may lack solid scientific backing, distorting public understanding and fostering unease. The complex interaction between media influence, public opinion, and policy responses underscores the need for improved education and clearer communication about GMOs to better align public perceptions with established scientific results.47
Consumers tend to place greater trust in scientists and academic institutions over media, industry, or governmental sources, viewing academic sources as more objective and thorough (Figure 8).48 Nevertheless, there remains strong demand for transparency and mandatory GMO labeling, which is seen as vital for enabling consumers to make informed choices. This demand is particularly strong in regions such as the United States and the European Union, where public pressure and policy actions have significantly influenced labeling regulations.49
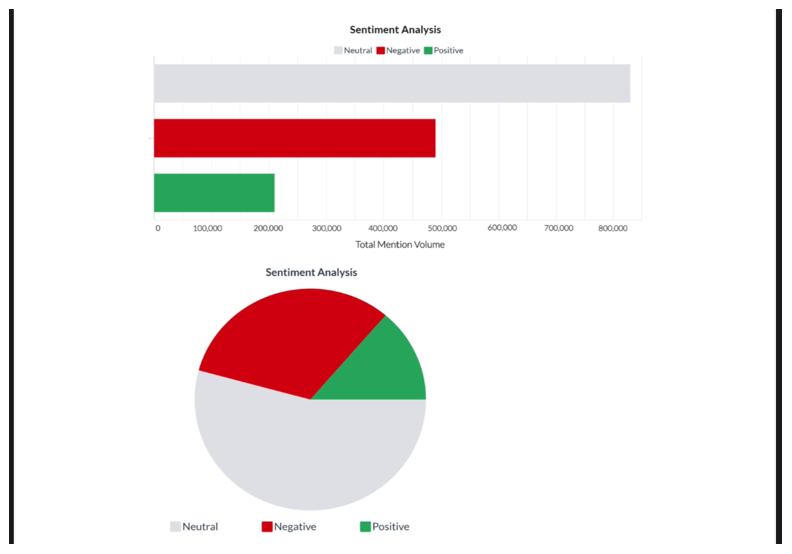
Figure 8 Sentiment analysis of social media mentions regarding GMOs.
This figure displays a sentiment analysis from social media, categorizing mentions of GMOs into neutral, negative, and positive sentiments, as shown in the bar graph and pie chart. The data, extracted via Brandwatch software, highlights a predominance of negative sentiments, illustrating public skepticism influenced by media portrayals of GMOs.
Advanced genome editing techniques
Advanced genome editing techniques such as CRISPR/Cas9, prime editing, and base editing are rapidly transforming crop improvement efforts by enabling precise genetic modifications. CRISPR/Cas9, a foundational tool in genome editing, introduces targeted DNA breaks, facilitating gene knockout or the introduction of desired traits.50 Recent advancements have led to prime editing, which avoids double-strand breaks and allows for accurate insertion, deletion, and base-to-base conversions, significantly reducing off-target effects and increasing specificity in plants like rice and Arabidopsis.51 Additionally, base editing enables single-base alterations without inducing double-strand breaks, expanding the potential for multiplex genetic changes critical for crop enhancement.52
Automation and artificial intelligence in tissue culture
Automation and artificial intelligence (AI) are transforming plant tissue culture, markedly improving efficiency, precision, and scalability. These technologies enable precise control over growth conditions and automate routine tasks such as micropropagation and explant handling, reducing labor requirements and minimizing human error.53 Moreover, AI algorithms analyze extensive datasets to predict optimal growth outcomes and dynamically adjust protocols, enhancing the adaptability and efficiency of tissue culture practices.54 For instance, robotic systems now perform delicate operations such as cutting and transplanting tissue cultures, ensuring consistent handling and reducing contamination risks (Figure 9).55 These innovations not only streamline production but also significantly reduce costs, making advanced tissue culture techniques more accessible and economically viable. As AI and automation continue to evolve, they are setting new industry standards for producing high-quality, genetically uniform plantlets, and supporting scalable and sustainable agricultural operations.56
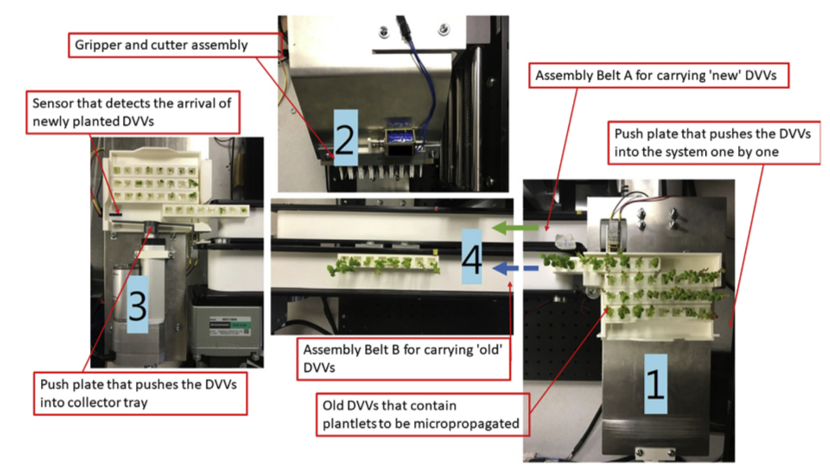
Figure 9 Automated micro propagation system.
This figure depicts an automated micro propagation system, showcasing key components: feed-in compartment (1) for inputting plantlets, the main compartment (2) with precision gripper and cutter assembly, collector compartment (3) for output, and assembly belts (4) for transport. The system utilizes robotics and AI to enhance efficiency, reduce manual labor, and set new industry standards for scalable, high-quality plant tissue culture.
Regulatory and ethical considerations
Ethical and regulatory considerations in agricultural biotechnology reveal diverse global perspectives on biosafety, public health, and environmental impacts.57 Different countries adopt unique regulatory approaches, with some enforcing strict controls on gene-edited and genetically modified organisms (GMOs) to protect biodiversity and address public concerns over potential ecological risks.58 Ethical debates frequently center on issues such as environmental sustainability, societal effects, and cultural or religious beliefs surrounding gene editing, all of which influence regulatory stances and public acceptance. Public perception plays a critical role in policy development, as consumer acceptance varies widely; for instance, the European Union mandates rigorous assessments for GM crops to address health and environmental concerns.59 These regulatory differences highlight the importance of developing balanced, globally aligned policies that foster both innovation and safety in advancing agricultural biotechnology.58
Synthetic biology and crop design
Synthetic biology is revolutionizing crop design by enabling the development of plants with enhanced resilience, higher productivity, and improved nutritional value.60 This approach integrates engineering principles into biological systems, allowing for precise genetic modifications that extend beyond the capabilities of conventional breeding. Through the design of genetic circuits and metabolic pathways, synthetic biology makes it possible to create “super crops” that can withstand environmental stresses, increase photosynthetic efficiency, and reduce reliance on fertilizers.61 Advanced computational tools and gene-editing technologies, such as CRISPR, play a crucial role by enabling scientists to incorporate complex, multi-gene traits into plants, tailoring crops to specific agricultural needs and environmental conditions.62 Additionally, synthetic biology facilitates targeted modifications of metabolic pathways to produce health-enhancing compounds, expanding the potential of crops to address nutritional challenges and promote sustainable agriculture.63
This review has critically evaluated the transformative advancements in plant tissue culture and genetic transformation, underscoring their pivotal role in enhancing agricultural biotechnology.3 Techniques such as CRISPR/Cas9 genome editing and synthetic biology have revolutionized traditional farming methods by enabling precise genetic modifications that improve crop yields, enhance nutritional profiles, and increase resistance to environmental stresses. These innovations not only facilitate the rapid propagation of high-yield, disease-resistant plants but also promote sustainable agricultural practices by reducing reliance on chemical inputs such as fertilizers and pesticides.7
Despite these technological breakthroughs, integrating these advancements into mainstream agricultural practices poses considerable challenges. Public skepticism toward genetically modified organisms (GMOs) persists as a significant barrier, driven by concerns over potential health impacts and ecological risks.41 The regulatory landscape governing the use of GMOs also presents complexities, varying significantly across countries and often hindering the adoption of these technologies. Ethical concerns further complicate this issue, as debates continue regarding the morality of altering natural organisms at a genetic level.46
Addressing these challenges is critical and requires robust research, transparent communication, and comprehensive regulatory strategies to enhance the efficiency and safety of genome-editing tools. Developing international regulatory frameworks that can adapt to rapid technological advancements while ensuring environmental safety and public health is essential. Moreover, fostering constructive dialogue with the public to enhance understanding and clarify the benefits and risks associated with GMOs is crucial for building trust and achieving broader acceptance. By effectively navigating these issues, plant tissue culture and genetic transformation can realize their full potential, significantly contributing to global food security and promoting sustainable agricultural practices.47
There is no funding to report for this study.
Pavan Koti expresses appreciation to Professor Bill Tawil for overseeing the framework of this review, Plant Tissue Culture and Genetic Transformation in Crop Improvement, and for the insightful lectures and advice concerning directed studies, which contributed significantly to the development of this paper.
The authors declare that there are no conflicts of interest.

©2025 Koti, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.