Journal of
eISSN: 2469 - 2786


Review Article Volume 13 Issue 1
Department of Life Sciences, Maharaja Krishnakumarsinhji Bhavnagar University, India
Correspondence: Anjana K Vala, Department of Life Sciences, Maharaja Krishnakumarsinhji Bhavnagar University, Bhavnagar- 364001, India
Received: November 30, 2024 | Published: January 7, 2025
Citation: Dave J, Maniyar R, Makwana P, et al. Phosphate solubilizing microorganisms from hypersaline environment: a review. J Bacteriol Mycol Open Access. 2025;13(1):1-6. DOI: 10.15406/jbmoa.2025.13.00389
Various aspects of phosphate solubilization are discussed including mechanism growth of halophilic plants; promotion of microorganisms isolated from saline environment near Bhavnagar coast, Gujarat, India focusing on their phosphate and its solubilization ability. Problems related to salinity ingress and nutrient deficiency are discussed especially remediation of phosphorus deficiency. Role of halo tolerant or halophilic microorganisms are revealed and effective capabilities of phosphate solubilization offering potential as bio fertilizer for enhancing plant growth in saline/hypersaline.
The research promotes sustainable agricultural practices that are consistent with Sustainable Development Goals such as "zero hunger," "sustainable consumption and production," and "life on land."
Keywords: halophilic microorganisms, phosphate solubilization, sustainable agriculture, saline and hypersaline environments
Increasing population is directly connected with biodiversity loss, ecosystem collapses, increasing poverty, resource depletion, and an increase in hunger and it’s required to take steps for global food demand, sustaining soil fertility and improving crop yield in agriculture field. For sustaining soil fertility, availability of nutrients in soil which helps in plants growth promotions (PGP) more precisely, availability of nutrients nitrogen (N), phosphorus (P), and potassium (K) to plants is crucial. Decreasing soil fertility, particularly P depletion, is a major issue affecting plant growth and crop production all over the world as P is second only to nitrogen among mineral nutrients most common limiting growth factor for plants, as about 0.05-0.5% of a plant’s dry weight comprises P.
Many microorganisms are capable of solubilizing insoluble phosphate to release soluble phosphate to plants. These microorganisms play an important role in improving yield of agricultural crops. Although salinity exposure is one of the BAD FACTORS affecting yield, phosphate-solubilizing microbes (PSM) from saline and hypersaline environments play important role in tackling the problem.
Microorganisms including bacteria and fungi have several mechanisms that allow the mineralization of organic P and solubilization of the inorganic P unavailable to plants.1–3 Halophilic/halo tolerant microbes have been isolated from diverse hypersaline environments such as solar salterns, hypersaline lakes, the Dead Sea, hypersaline microbial mats, and underground salt deposits.4 While salinity ingress is a major issue worldwide, such microbiota could be potentially harnessed in saline ecosystems for promoting plant growth.
This review presents a comprehension on research about phosphate solubilizing microorganisms and a case study of phosphate solubilizing bacteria and fungi from hypersaline environment has also been discussed.
Soil salinization is a significant abiotic factor threatening agricultural production, while the low availability of phosphorus (P) in plants is another worldwide limitation.4 Approximately 95-99% of the P in soil is unavailable to plants. Total P content in soil is around 0.05% (w/w), out of this only 0.1% P is accessible to plants.5–7 Majority of P is available in soil is in inaccessible form to plants thus there is only about 30-40% land which favours plant cultivation.8 The phosphorus cycle is considered as a “sedimentary cycle” and in soil P is available mainly in three forms depending on its solubility and chemical nature, such as (i) insoluble inorganic phosphate (Pi);(ii) soluble orthophosphate (bioavailable phosphate); and (iii) insoluble organic phosphate (Po). Generally, insoluble Pi exists in the form of more stable primary P minerals (apatites, strengite, and variscite), secondary P minerals (e.g., Ca–P, Fe–P, and Al–P), and sorbed P (e.g., in clay and in Fe and Al oxides) in soil. The insoluble Po, constitutes around 30% to 65% of the total phosphorus content and it exists in various states, including inositol phosphate (soil phytate accounting for 50% of total organic P), phosphomonoesters, phosphodiesters, which encompass phospholipids, nucleic acids, and phosphotriesters. Depending upon the pH condition the soluble orthophosphate exist in three forms viz; Mono-Hydrogen Phosphate (HPO42-), Di-hydrogen Phosphate (H2PO4-), and Tri-Hydrogen Phosphate (H3PO4) and plant might mainly use HPO42- & H2PO4-. Phosphorus undergoes to its different forms through natural processes involving physical and chemical mechanisms, these include weathering, desorption-adsorption, dissolution-precipitation, and immobilization. Additionally, microbial activities, such as solubilization of insoluble phosphate (Pi) and mineralization of insoluble organic phosphorus (Po), this contributes to the complex phosphorus cycling within ecosystems. P accessibility depends on several factors such as soil pH, temperature, moisture, organic matter, metal oxides, clay minerals and it is also known that increasing salinity in arable soil also severely affects the P availability, which is needed to be taken into consideration.9
Environmental stress lead to decrease in various microbial activities such as respiration, nitrogen mineralization, and the functioning of various enzymes.10 An increase in salinity can impact the efficacy of phosphate solubilizing microorganisms due to the inhibition of certain enzyme activities.11 For instance, the activities of enzymes such as dehydrogenases, which play a crucial role in acid synthesis, and phosphatases, involved in the mineralization of organic phosphorus, can be inhibited as a result the overall performance of microorganisms in phosphate solubilization can be severely compromised. Salt can decrease the activity of several enzymes such as dehydrogenases and phosphatases.
Many of plant species cannot tolerate high salinity. The excess salt can cause harmful effects related to osmotic and ionic stress, which globally affect the plant and also impair its water imbalance and nutrition, leading to a reduction in the photosynthetic rate and the generation of reactive oxygen species that cause molecular damage. However, some plant species, called halophyte plants, have adapted to environments with high salinity.12 These adaptations can be morphological, physiological, or biochemical but can also be related to symbiotic interactions with plant growth-promoting bacteria (PGPB).13
Salinity ingress is a major threat to agriculture, food security and economic development causing a reduction in net arable land (such as arid, semiarid and irrigated land) as of now about 23 million acres of arable land is affected by salinity ingress worldwide, in India about 6.37Mha area is salt-affected covering many states and it is even estimated that 50% of the arable land will be affected by salinity by 2050.14–18 Soil salinity is classified in to three groups on the bases pH, EC (electrical conductivity) , and ESP (exchangeable sodium percentage). So, soil with pH <8.5, EC >4 dS/m and ESP<15 is saline soil, a soil with pH>8.5, EC >4 dS/m, and ESP >15 is saline sodic soil, and a soil with pH >8.5, EC <4 dS/m, and ESP >15 is a sodic soil.19 High salinity affects the plant development at all stages even in reproductive development, seed germination, vegetative growth by affecting (i). Water availability to plants, (ii). Photosynthesis through reduction in chlorophyll content and leaf area, stomatal conductance, decrease in PS II efficiency (iii). Imposing low osmotic potential, ion toxicity and nutritional deficiency (mainly N, P, and K). This all hinders the plant growth hence new strategies has to form to tackle salinity ingress for sustaining soil fertility and sustainable agriculture.20–24
In order to overcome the problem of plant growth in salinity stressed arable land and nutrient deficiency especially P deficiency, phosphorous fertilizers are used. Many commonly used fertilizers for crops contain phosphorous that is not readily available for plant uptake and its excessive usage can lead to environmental problems such as eutrophication and groundwater contamination.25,26 Hence there is a need of using biological fertilizers which significantly does not cause any environmental problems for example NPK bio fertilizer which generally contain plant growth promoting microorganisms which are capable of fixing nitrogen, solubilizing phosphate and mobilizing potassium which significantly reduces the need for chemical nutrient additives, resulting in healthy plants, abundant crops and lower input cost.
Phosphate solubilizing microorganisms play an important role in P cycling in the soil.26 These microorganisms make P available to plants and act as plant growth promoters. The mobilization of P by microorganisms occurs through a variety of mechanisms A) acidification of the medium by extrusion of H+ and/or organic acids B) metal complexation C) metal reduction D) extrusion of phosphatases and E) indirect dissolution of phosphate through the stimulation of acid production by plants. Many studies have sought to isolate and identify phosphate solubilizing bacteria as bio prospecting strains with the potential to develop alternative for P management in agriculture27,28 Many of the microorganisms mobilize P, but their transformation capacity may be associated with ecological conditions.
The plant growth-promoting characteristics involve processes like fixing nitrogen from the air, 1-aminocyclopropane-1-carboxylate (ACC) deaminase activity, solubilization of nutrients like zinc, potassium, and phosphorus, creating substances like ammonia, hydrogen cyanide, siderophores (which help plants absorb iron),29-32 and plant hormones such as cytokinin, gibberellic acids, and indole acetic acids.33,34 Additionally, these processes also lead to the production of other useful compounds known as secondary metabolites. The rhizosphere is an area around plant roots were various organisms like bacteria, fungi, and even algae live and play role in promotion of plants growth this are termed as plant growth promoting rhizobacteria (PGPR).35-39 Salt tolerating PGPR (ST-PGPR) could be the breakthrough to overcome the salinity effect on plant at salinity ingress area as this microbe of various genera are reported for enhancing plant survival and its growth in saline environments by means of their plant growth promoting characteristics as mentioned above and these organisms could be used as bio inoculants instead of chemical-based fertilizers.
Salt-tolerant or halophilic soil microorganisms are able to dissolve insoluble phosphates, thereby contributing to the development of agriculture on saline and alkali soils. The ability to solubilize phosphates was characterized in detail for mycorrhizal fungi40 that are also proven to contribute to the crop nutrition by increasing the volume of rhizosphere and thus the volume of soil from which phosphates can be absorbed41 Other soil microorganisms are phosphate-solubilizing bacteria (PSB) that are generally associated with the plant rhizosphere.42 According to the literature data, PSBs account for 1–50% of the total microbial population, compared to the phosphate-solubilizing fungi accounting for 0.1–0.5%. Phosphate-solubilizing microorganisms are ubiquitous, with their species composition varying from soil to soil. Many of the phosphate-solubilizing microorganisms have been isolated from the rhizosphere of various plants, where they are metabolically active.43
The plant microbiomes have been reported as epiphytic, endophytic, and rhizospheric and have been characterized for plant growth promotion in saline condition. Archaea is one of the most abundant microbes reported from extreme saline and hot springs environments and there are few reports on archaea as associated with plants such as maize, rice, and halophytic plant. Different mechanisms have been used for phosphate solubilization by the actions of microorganisms mostly eubacteria and fungi. Halophiles are organisms that thrive in high salt environments by balancing the osmotic pressure of the environment in order to resist the denaturing effects of salts. There are many novel species of halophilic microbes isolated from crops growing under saline conditions, saline lakes and hypersaline soils (Actinopolyspora mortivallis, Halothermothrix Orenii, Natrinema versiforme, Halomonas marisflavae, Bacillus marisflavi, Arcobacter halophilus, Nesterenkonia aethiopica, Aquisalimonas asiatica, Amycolatopsis halophila,44 Pontibacillus yanchengensis,45 Streptomyces chilikensis,46 Prauserella isguenensis,47 Marinirhabdus gelatinilytica,48 and Haloprofundus marisrubri. Along with halophilic microbes, the halophilic fungi such as Alternaria, Aspergillus, Cladosporium, Debaryomyces, Hortaea, Myrothecium, Penicillium, Piriformospora, Saccharomyces, Stemphylium, Sterigmatomyces, Trichoderma, Ulocladium, and Wallemia have been isolated from the diverse hypersaline environment and hypersaline lakes worldwide (Figure 1).44-47
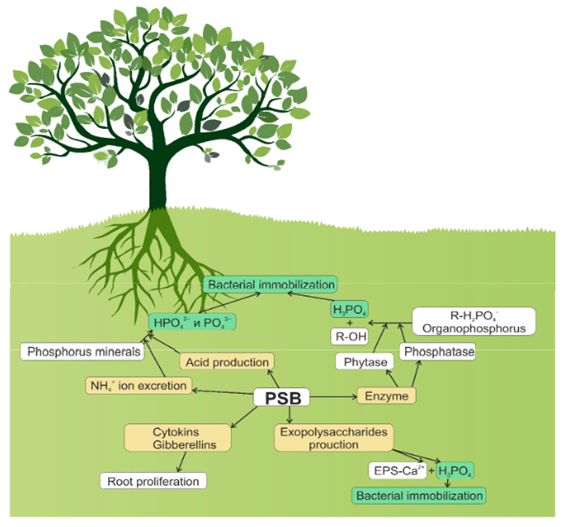
Figure 1 Role of PSBs in the solubilization of phosphates: solubilization of inorganic phosphates due to the synthesis of organic and inorganic acids; NH4+-excretion; solubilization of organic phosphorus-containing compounds due to the synthesis and excretion of enzymes; synthesis of polysaccharides, cytokinins, and gibberellins.48
During the biogeochemical recycling process, the phosphorus accumulated by bacteria and fungi is slowly released back into the soil and becomes available to plants, as evidenced by the correlation between phosphorus uptake by plants and phosphorus biomass in microorganisms.49 The rate of phosphate release from microbial biomass likely depends on the amount of phosphorus available in soil, carbon availability,50 soil texture, and composition of microbial community. The ability of microorganisms to balance between solubilization (mineralization) and immobilization processes determines the extent to which these bacteria and fungi can improve the availability of phosphorus in the soil–plant system.
This study mainly focuses on potential halophilic plant growth promoting bacterial and fungal phosphate solubilizing isolates obtained from an arid region near Bhavnagar coast, Gujarat, India, within the vast saltpans during the salt production month and are commonly referred to as salt-tolerating phosphate-solubilizing microorganisms (ST-PSM). Seven fungal isolates and two bacterial isolates were studied for their phosphate solubilization ability and this study provides valuable future prospects of using halophilic plant growth promoting organisms as bio fertilizers for sustainable agriculture in salinity ingress or salinity affected agricultural land. Understanding of microbial diversity and their biochemical activities in such extreme environments is crucial for biotechnological applications, valuable insights in ecology and agriculture. As salinity ingress is becoming threat for sustainable agriculture and in approaching future food demands with increasing population this study would help to achieve a few out of 17 Sustainable Development Goals by United Nation (2016) with the aim of peace and prosperity for people and the planet, for now and into the future such as “Zero hunger”, “Sustainable consumption and production”, “Life on land”.
Isolation of saltpan-derived microbes was carried out from Bhavnagar (21°46′N 72°09′E/21.77°N 72.15°E), Gujarat, India as described by Maniyar et al.50 Bhavnagar produces around 35,000 tons of salts annually which makes it the leading producer of salt in Gujarat. While 7 fungal isolates (RJ1, RJ2.... RJ7) were obtained using modified Czapek-Dox agar,50 2 bacterial isolates (RJB1 and RJB2) were obtained using modified LB(MLB) media.
Screening of phosphate solubilizers
The bacterial and fungal isolates were preliminarily screened for their phosphate solubilization ability by using Pikovskaya’s medium (PKVK) (Tri-Calcium Phosphate as insoluble inorganic source) amended with 3M salt. The PKVK agar plates were inoculated with fungal and bacterial isolates in duplicates. The inoculated plates were monitored for clear zones around colony. The test isolates exhibiting clear zone were further selected for testing their phosphate solubilization efficiency.
Determination of phosphate solubilization activity
Phosphate solubilization activity test was carried out using Stannous Chloride method. The fungal and bacterial isolates exhibiting clear zones in PKVK agar plates were further inoculated in 100ml sterile PKVK broth in duplicates in 250ml conical flask and were incubated at room temperature, uninoculated PKVK broth was kept as control. For each fungal isolate the 5ml filtrate withdrawal was carried out after 7th, 8th, 10th and 11th day of incubation after inoculation and each bacterial isolate’s 5ml withdrawal was carried out after 11th, 17th, 18th and 21st day of incubation after inoculation. Centrifugation at 5000rpm for 15 minutes was carried out to obtain bacterial supernatant. Filtrate/supernatant collected were further estimated for phosphorous presence using stannous chloride method. The estimation was also carried out in duplicates for confirmation of positive results and then the mean was taken.
Determination of salt tolerance and phosphate solubilization efficiency
From 7 fungal and 2 bacterial isolates the best fungal and bacterial isolate able to grow on >4M salt concentration on PKVK agar were selected and experiments were performed in triplicates (for 30days) to further ensure the consistency of phosphate solubilization ability using PKVK broth under hypersaline conditions and to see the temporal effect on phosphate solubilization.
Isolation of phosphate solubilizers
Among all test isolates, six fungal isolates i.e., RJ1, RJ2, RJ3, RJ4, RJ5, RJ6 and one bacterial isolate RJB2 exhibited clear zones on PKVK agar plates and were further selected for testing their phosphate solubilization efficiency. Selected fungal and bacterial isolate are shown in Figure 3.
Quantification of phosphate solubilization
Based on the agar plate results, selected isolates were examined for phosphate solubilization efficiency after inoculating them in PKVK broth (uninoculated PKVK broth was kept as control). Filtrates collected were subjected to estimation of phosphorus using stannous chloride method. The data are represented in Figure 4. As revealed by Figure 4, fungal isolates exhibited more phosphate solubilization than bacteria. Molecular identification based on 16s rDNA sequence of isolate RJB2 revealed it to be Salibacterium sp. strain LS-MKBU (Genbank: OR524060.1). Determination of phosphate solubilization activity at higher salt concentration and efficiency
Among all test isolates, fungal isolate RJ1 was able to grow on >4M salt concentration and was efficiently able to solubilize phosphate as well. The result obtained are shown in Figure 5. The isolate RJ1 is not only an efficient phosphate solubilizer but also an efficient L-asparaginase producer and its molecular identification revealed it to be Aspergillus unguis strain LS-MKBU (GenBank: OR405853.1).50
As shown in Figure 5, phosphate solubilization increased with increase in incubation period. More than fivefold increase in phosphate solubilization was observed this is a noteworthy observation suggesting a positive temporal impact. Without any further optimization process employed, such an increase was observed.
Solubilization of phosphate is a complex process influenced by a number of factors. Production of organic acids by microbes is among the most possible mechanisms for phosphate solubilization. The data, though preliminary, are suggestive of possible applicability of these microbial isolates as plant growth promoting microorganisms in salinity ingressed areas. These preliminary findings suggest especially isolate RJ1 Aspergillus unguis LS-MKBU) as potential candidate for phosphate solubilization. The isolate has displayed remarkable phosphate solubilization. To the best of authors’ knowledge, this is the first ever report on phosphate solubilization by saltern-derived microorganisms from Bhavnagar Coast. It suggests that halophilic microorganisms with phosphate solubilizing activity could impart plant growth promoting activity in saline/hypersaline soils. Optimization of phosphate solubilization, metabolite analyses from the broth and molecular identification of rest of the isolates are under progress in our laboratory.
Agriculture sustainability is one of the main pillars for sustaining mankind. While there are a number of issues (related to increased urbanization and industrialization) leading salinity ingress in agricultural soils, microorganisms from saline and hypersaline environment are envisaged as a solution to this pollution. Though, a few microbes have been revealed to exhibit plant growth promoting potential under hypersaline environment, the field demands further detailed explorations.
None.
The authors declare that there are no conflicts of interest.

©2025 Dave, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.