Journal of
eISSN: 2378-3184


Research Article Volume 14 Issue 1
National Scientific Center for Marine Biology, n.a. A.V. Zhirmunsky, Far Eastern Branch of the Russian Academy of Science, Vladivostok, Primorsky Territory, Russia
Correspondence: Gabaev DD, National Scientific Center for Marine Biology, n.a. A.V. Zhirmunsky, Far Eastern Branch of the Russian Academy of Science, Vladivostok, Primorsky Territory, Russia
Received: April 07, 2025 | Published: April 16, 2025
Citation: Gabaev DD, Kolotukhina NK. Gastropod shell Nucella (Thais) Heyseana -predator at the mariculture Objects. J Aquac Mar Biol. 2025;14(1):51-59. DOI: 10.15406/jamb.2025.14.00415
Seafood farming (mariculture) is one of the fastest growing industries in the world. However, the cultivation technologies are not perfect yet, which does not allow them to achieve profitability everywhere. The article presents the results of population dynamics, spatial distribution, dietary variability, food digestibility, growth rates and the degree of impact predatory gastropods - Nucella heyseana (N. heyseana) on bivalves. The relationship between the size of N. heyseana and the victims as well as the degree of its influence on the surviving molluscs grows is clarified. The holes’ location on the shells of dead mussels Mytilus trossulus (M. trossulus) and oysters Magallana (=Crassostrea) (M. gigas) made it possible to determine the places most susceptible to drilling. It has been established that N. heyseana has a short pelagic stage in its development which allows it to colonize collectors and cages used for M. yessoensis breeding. A contents’ comparison of scallop collectors with and without N. heyseana showed that as a rule there were two more bivalve species with predator then without it. There are mainly Chlamys farreri (Ch. farreri) and Musculista (M. senchousia). Five months later after the metamorphosis in collectors without N. heyseana the death of Mizuhopecten yessoensis (M. yessoensis) and Ch. farreri was observed. The eating of the mussel M. trossulus by N. heyseana - a competitor of M. yessoensis scallop improves its living conditions and increases the cultivation efficiency.
Keywords: positive effect of the predatory gastropod Nucella heyseana on cultivated scallop Mizuhopecten yessoensis.
In the cultivation process of the Japanese scallop M. yessoensis (Jay) and the Pacific mussel M. trossulus (Gould) in Minonosok Inlet of the Posyeta Bay (42º61ʼN, 130º86ʼE) and in whale Inlet (43º06ʼN, 134º20ʼE) on artificial substrates we have discovered the predatory gastropod N. heyseana (Dunker, 1882). The empty shells of bivalve mollusks with characteristic holes with a diameter of 1-2 mm were also found there. The artificial substrates - the scallop collectors and cages are located deep in water and the appearance of the N. heyseana in them can be explained by the presence of a pillaging period in its development. But most researches of this field declare that Nucella (= Purpura = Thais = Polytropa) does not have a pelagic stage and the juveniles emerging from the capsules immediately begin to crawl.1-4 The significant influence made by N. heyseana on M. trossulus in natural laboratory conditions,5-8 and also its capacity to switch from one victim species to another.2,9,10 require deep research of the N. heyseana ecology. The present research is devoted to the ecology issues and the N. heyseana larval development clarification.
To research the population dynamic of N. heyseana, M. yessoensis, M. trossulus and Asterias amurensis (A. amurensis) the contents scallop collectors of Japanese construction and developed by us collector-cages in Minonosok Inlet and in Klykova Inlet of Posyeta Bay (42º61ʼN, 130º85ʼE) were studied during 38 years (1977 – 2014) (Figure 1).
The cages' inhabitants were also studied one year after settling in them one-year-old M. yessoensis. During four years the same research was carried out in whale and Trinity Inlets (42º64ʼN, 131º11ʼE) (Figure 1). The scallop collectors that had overwintered at sea were processed in the spring of the following year and a small batch was left for another year. The cages with M. yessoensis were introduced in the sea for one year. In shallow bays they were lowered to a depth of 6-1o m and in open areas up to 20 m. In 1988-1990 the scallop collectors were immersed in Minonosok Inlet dynamically - two garlands every other day from late May to early July. In September - November three collectors were removed from each garland and all the mollusks and the A. amurensis were extracted. Live and dead individuals were counted. The results were recalculated per 1 m2. In 30-50 individuals of each species (if fewer were found then all of them), the height of the shells was measured with a caliper with an accuracy of 0.1 mm. From 1987 to 1996 the mollusks waited on a VLTK-500 scale with an accuracy of 0.01 gr. The total body weight (W total, gr.), shell weight (W shell, gr.), soft body weight (W meat, gr.) and the dry mass of meat dried at 600 C to a constant weight (W dry meat, gr.). The meat weight of M. trossulus was determined according to.11 The age of N. heyseana was determined by growth rings12,13 and by the time when the constructions were in the sea. The Bertalanffy equation was used as a linear growth model:
where Lt = the shell height (mm) at the age t (years),
L∞ = the theoretical maximum shell length,
к = the constant characterizing the rate of deceleration growth,
t0 = the age at which the shell length is zero.
The values of the parameters L∞, k and t0 of equation (1) were determined by the Walford method14 with the use of analytical techniques. The life length D was found using the formula15:
where L max = maximum shell size in the researched population. The food selectivity at N. heyseana was determined by:2 but the diet range by the preference index α16,17:
where i = victim type,
nio = a number of victim types i represented,
ri = a number of victim types i consumed.
For four different-sized individuals of N. heyseana the preference histogram was constructed for four different-sized groups of M. trossulus. An idea of diet selectivity was obtained by comparing the numerical structure of living and dead M. trossulus.
To observe the food activity in the aquarium 108 individuals of two-year M. trossulus were added to 16 individuals of N. heyseana in December 1996. In spring of 1997 in another aquarium with a bowl of sludge 3 specimens of two-year-old M. trossulus were placed with 9 individuals of N. heyseana. During the subsequent two-year stay of N. heyseana in aquariums they were provided with M. trossulus taken from the collectors. Each aquarium had a volume of 140 liters and the water there was changed every other day. The aeration in the aquariums was constant. After N. heyseana spawning one capsule was removed from the clutch daily and the larvae embryonic development was observed under MBS-9 and “Docuval” microscopes. The juveniles emerging from the clutch were photographed using a micro photo attachment. To study the juveniles' feeding activity, four cylinders with a base area of 0.75 cm2 floating at the surface of the water were placed in the aquarium, the bottom of which was covered with a nylon sieve with a mesh of 200µm. Each cylinder contained about 40 N. heyseana larvae, one of which contained a small M. trossulus (up to 7mm in valve height), and the other a one-year-old gastropod Epheria turrita (E. turrita). The other two cylinders contained juvenile of N. heyseana with either empty capsules or without capsules. The experiment lasted five months from the moment the clutches were laid until the complete death of young N. heyseana.
To feed M. trossulus a microalgae was added to the aquarium and during the water change all dead mollusks were collected. The shell height, the hole diameter, the hole location and the shell of M. trossulus was measured. The mass of dead M. trossulus was determined by the previously established ratio between shell height and the weight of soft and dry tissues. The values of salinity and surface water temperature, wind speed and direction, sea level and precipitation in Posyeta Bay in June 1977-2014 was obtained by the weather station in the Posyet village (42°39’ N, 130°48’ E) were provided to the authors employee of FEB RAS Ph.D. Rostov I.D. Statistical analysis of the material was carried out using the STATISTICA 6 program18 and tasted at the level of α = 0.05.
The results of observation in natural conditions.
On natural substrates N. heyseana is found in the most surfy part of bays. As a rule these are rocky capes where other predatory invertebrates cannot penetrate because they are dislodged by wave action, while N. heyseana has a smoother shell and is able to live above sea level. The water area where N. heyseana is colder compared to the shallow, well-warmed part of Novgorodskaya (42º64ʼN, 130º90ʼE) and Expedition (42º66ʼN, 130º75ʼE) bays. In this bays the larvae of N. heyseana do not settle on collectors and the adult individuals can easily be found on the capes bordering to Minonosok, Klykova, Postovaya (42º65ʼN, 130º80ʼE), Reid Pallada (42º59ʼN, 130º84ʼE), Trоitsa Inlet of Posyeta Bay and in the cold-water Kit bay (Figure 1). In Minonosok Inlet N. heyseana settles on flat rocks colonized by M. trossulus.
Despite the abundance of mariculture plantations in Minonosok Bay, it is difficult to detect live M. trossulus on the bottom due to their poor resilience. M. trossulus can sometimes be found under the plantations on fallen artificial substrates and near the pier, where mollusks settled in scallop collectors are sorted. At the bottom N. heyseana has competitors - several species of starfish, gastropods, sea urchins, fish and birds. They eat fast M. trossulus and in littoral M. trossulus die in winter due to sea level falling and the abrasive ice effect. In summer the stocks of victims run out and N. heyseana has cases of cannibalism and mass death. At the end of October 1986, we found 19 specimens of dead N. heyseana on the 5-meter shore at Cape Lowland (42º60'N, 130º87'E) and five of them had holes from their relatives.
N. heyseana began to occur frequently at the bottom and the first time it appeared on artificial substrates 6 years after the start of industrial cultivation of the mussel M. trossulus in 1979 in the Minonosok Inlet. The density of N. heyseana at the bottom was constantly increasing and by the summer of 1996 reached 2,200 ind./m2 with biomass up to 6.9 kg/m2. However, on artificial substrates, the density of N. heyseana did not exceed 0.2 ind./m2 (Figure 2), and they settled in scallop collectors that were submerged in the sea from May 25 to June 8. N. heyseana was found at depths of 0-17 m, but more often at 6-8 m. The maximum abundance of N. heyseana in scallop collectors quite often coincided with an increase in the abundance of M. trossulus (Figure 2) and 19-year observations showed that the Pearson correlation coefficient between their dynamics was significant (r = 0.556: p = 0.014).
The equation of abundance dependence of N. heyseana on M. trossulus has the form:
Y = 0,008481 + 0, 000022 x (R2 = 0,104; p< 0,07)
where х is the abundance of M. trossulus (ind./m2 of collector), and Y is the abundance of N. heyseana (ind./m2 of collector). At the same time, an inverse relationship is observed between the dynamics of the abundance of another predator - the starfish A. amurensis and M. trossulus (Figure 3).
The equation of dependence of the abundance of A. amurensis from M. trossulus has the form:
Y = 2, 194 - 0,00099 x (R2 = 0,154; p< 0,027)
where x is the abundance of M. trossulus (ind./m2 of collector), and Y is the abundance of A. amurensis (ind. /m2 of collector). After abandoning the cultivation of M. trossulus in the Minonosok Inlet in 1989, the abundance of M. trossulus began to decrease, and already in 1991 A. amurensis began to occur 4 times more often, and N. heyseana disappeared and between them there is an opposite dynamics of the population size (Figure 4).
However, in 1994, M. trossulus cultivation was again attempted in Minonosok Inlet and already in June 1996 the abundance of N. heyseana reached high values on the bottom, and its juveniles began to occur in marine plantations overgrown with M. trossulus (Figure 4). At the Voevoda Inlet Marine Farm (43º 00 N, 131º78 E) where M. trossulus has been cultivated for many years, abundance of N. heyseana in 2024 reached 1.0 ind./m2.
In scallop collectors, a fairly obvious relationship is found between the harvest of the year and the number of M. trossulus eaten (Figure 5).
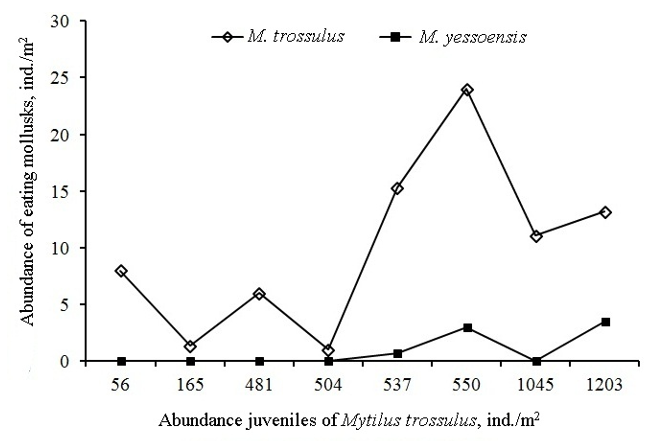
Figure 5 Variability of consumption of M. trossulus and M. yessoensis by one N. heyseana depending on the abundance of M. trossulus in the collectors.
The same is observed with M. yessoensis. In the harvest year of 1988, the settling of M. yessoensis larvae in the collector - cages of Klykova Inlet was 1025 ind./m2, and the death rate from one N. heyseana for a year and 2 months was 0.24%. However, in the non harvest year of 1989, the settling of M. yessoensis larvae in the collector - cages of Klykova Inlet was 300 ind./m2, and the death rate from N. heyseana for a year and 2.5 months was 0%. Large individuals of N. heyseana prefer large individuals of M. trossulus and M. yessoensis (Figure 6).
This is also confirmed by comparing the diameter of holes in M. trossulus with the height of their shells in collectors (Figure7).
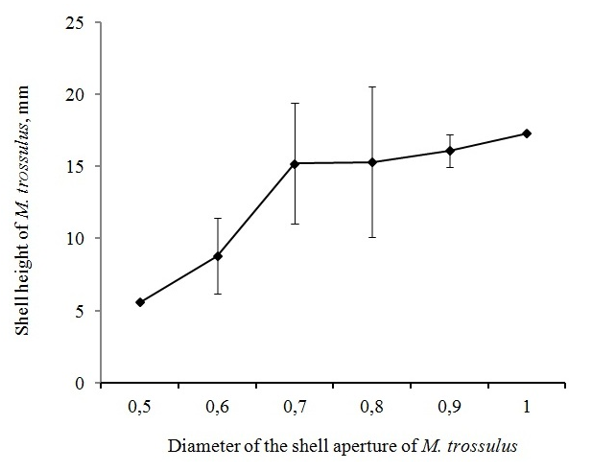
Figure 7 Relationship between the size of M. trossulus and the diameter of the hole in the shell from N. heyseana.
The correlation coefficient between the size of the shell of M. trossulus and the diameter of the hole from N. heyseana is 0.555 at p = 0.061. At the same time, comparison of the size of surviving M. trossulus in collectors with and without predators sometimes contradicts this pattern. In the presence of predators, surviving M. trossulus sometimes reach larger sizes than without predators (Figure 8).
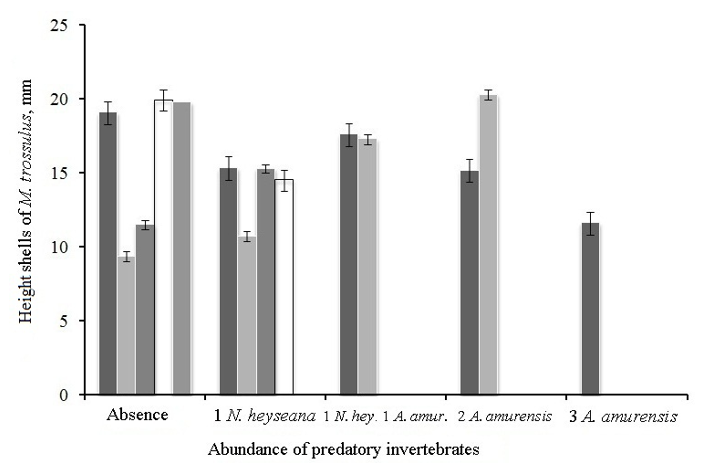
Figure 8 Shell height of M. trossulus survivors with different abundance of predators in the collectors.
In the summer of 1996, an overwintered population of M. trossulus was discovered in the offset zone of Troitsa Inlet, which N. heyseana fed on. The holes on the shell were more often located at the junction of the winter and spring growth zones. The eaten M. trossulus were larger than the live ones. Their shell length was 19.80 + 0.57 mm and 16.19 + 1.06 mm, respectively. The criteria of t-Student of these two samples showed a significant difference (t = 3.29; p = 0.002).
In November 2001, many juvenile oysters of Magallana (=Crassostrea) (M. gigas), which N. heyseana fed on, were found on the littoral of the Minonosok Inlet. The average diameter of the hole from it was 0.853 + 0.142 mm and the surviving (M. gigas) had a shorter shell length compared to the dead ones (10.41 + 0.36 mm and 11.07 + 0.41 mm, respectively). However, the criteria t-Student of these samples showed an unreliable difference (t = 1.21; p = 0.23). The holes from N. heyseana were more common near the umbo (45.16%) and in the area of the closure muscle (32.26%).
The research of four different-sized N. heyseana individuals' food preferences showed that small and medium-sized predators consumed large prey, but the largest predator ate small M. trossulus (Figure 9).
Of the variety of bivalves in the collectors, N. heyseana tended to attack M. trossulus (Figure 10).
However, in case of settling into the collector - cages, divided by plates into platforms with a volume of 0.1 m2 and having eaten M. trossulus there, switched to M. yessoensis. If the year turned out to be fruitful for the young scallop Ch. farreri, they switched to it despite its shells with thorns.
A comparison of the contents of collectors with and without N. heyseana showed that in collectors with a predator, as a rule, there were two more species of bivalves. These are mainly Ch. farreri and M. senchousia. In collectors without N. heyseana, the full death of M. yessoensis and Ch. farreri was sometimes observed already in late summer.
In scallop collectors, the number of M. trossulus is greater than on the bottom, so after 2.5 years of life, the shell height of N. heyseana was almost twice as high as that of individuals living on the bottom (Figure 11).
In the littoral of the middle part of the Primorsky Territory (whale Inlet) M. trossulus is easy to find and predatory sea stars A. amurensis and Distolasterias nippon do not occur there. This is probably why M. trossulus occurs more frequently under algal plantations and remains alive longer. Due to the abundance of food and lower temperatures, which lengthen the life span, N. heyseana reaches a record shell height of 78mm there and 77mm in the Minonosok Inlet collectors. The calculation of the eaten mass of M. trossulus and the weight gain of N. heyseana on artificial substrates allowed us to establish that the digestibility of its food in the collector - cage is 63.6%, and in the cage with a scallop - 50.3%. In the collector, to increase the diameter of the "trunk" by 0.1 mm, the N. heyseana had to eat an average of 3 M. trossulus specimens.
The results of the maintenance of N. heyseana in the aquarium
In a large sample of M. trossulus (n = 2140), it was found that there was no positive relationship between the hole diameter from N. heyseana and the shell size of M. trossulus (r = 0.0902; p = 0.000). However, the preference of N. heyseana for large individuals of M. trossulus is proved by the fact that large individuals were more destroyed than small ones. In addition, a highly reliable relationship was found between the size and number of holes from N. heyseana on one of M. trossulus (r = 0.974. p = 0.000 (Figure12).
A negative relationship was found between the thickness of the shell at the drilling point and the diameter of the hole in M. trossulus (r = - 0.060; p = 0.005) and most often N. heyseana drilled the thin-walled dorsal or posterior parts of the shell (24.9 and 20.8%, respectively) (Figure 13).
At the ventral edge of the shell at five drilling points, the total thickness of the flaps was 4 mm, and at the dorsal edge – 3.65 mm. Incomplete holes (0.33%) were found in 7 M. trossulus specimens out of 2,140 and they were located above the thick part of the shell. With a similar shell thickness and hole diameter, shells with incomplete holes were larger (L = 26.1+ 5.6 mm and L = 21.8+0.2 mm, respectively).
Without food, N. heyseana had cases of death, cannibalism and carrion feeding, and sometimes drilling began inside the deceased M. trossulus. However, in the presence of silt at the bottom, death and cannibalism were not observed, since N. heyseana often burrowed into it and remained motionless for a long time, possibly feeding on detritus. They did not grow for 9 months, and in the summer, after the introduction of M. trossulus, a compensatory growth was found. However, their growth rate lagged behind those living in the natural environment (Figure 11).
N. heyseana sensed the appearance of food at a distance and 10-12 hours after the appearance of M. trossulus, N. heyseana was encountered on them. At the same time, they covered a distance of 65 cm. The victims also feel the presence of predators, since the placer of M. trossulus was already group by the time of N. heyseana approach. Because of this, predators drilled holes in the unprotected part of the shell and almost half of the holes (47.7%) were made by N. heyseana at the dorsal edge.
With the onset of spring, the amount and total weight of M. trossulus consumed increased. By March 19, they reached their maximum values, and then sharply decreased (Figure 14). At that time, N. heyseana formed groups of 5-6 individuals and on M. trossulus hunted only single individuals. Some N. heyseana deposited capsules on the walls of the aquarium, and by the end of April, the feeding intensity of N. heyseana increased (Figure 14).
Compared with the natural environment, the digestibility of food in the aquarium reached a maximum of 65.5%.
At a temperature of 13° C, N. heyseana began deposited capsules in mid-December and the laying completed in the surface layer of the aquarium on February 17, 1998 subsequently turned out to be the largest. During the day, the individual interrupted spawning, crawled away, copulated with another individual and returned back. 108 spindle-shaped capsules 8-13 mm high with 25-60 eggs were laid. Their development lasted 35 days and during this time, 14-30 eggs of feeders were eaten in each capsule and 11-30 larvae entered the water. They swam inside the capsule and immediately after exiting it, then crawled, and later released the sail and began to swim again. Other individuals, after leaving the capsule, were attached to the surface of the water with the sole of their foot for several hours. The duration of the pelagic period was influenced by the temperature of the water. At 8° C, it lasted about a day, and at room temperature it was reduced to 4 hours. However, even a month after settling on the substrate, N. heyseana is able to float to the surface of the water and stay in the water for about a day.
With a four-year content with an excess of food, the number of deposited capsules increased to 610 pieces, and the number of emerging larvae increased to 19.8 + 9.7 specimens. They came out of the clutches with a small supply of yolk and went without food for a while. However, on the fourth day, the shells of an almost one-year-old M. trossulus were drilled and one individual was eaten in four days. Cannibalism was observed in cylinders without food, and up to 5 through holes in the shell were found in the dead N. heyseana and Epheria turrita (E. turrita). In a cylinder, thanks to the abundance of food N. heyseana grew intensively and in a month overtook the one-year-old E. turrita in shell height (3.5 and 3.0 mm, respectively).
Using our observations of the daily nutritional requirements of N. heyseana and determining the average meat weight of recently settled M. trossulus larvae, we found that one individual of young N, heyseana per day is able to eat 5.6 specimens M. trossulus Therefore, in five months, by the time the contents of the collectors are examined, the N. heyseana can eat 840 specimens M. trossulus larvae. These calculated data are consistent with the actual difference in the abundance of juvenile M. trossulus in collectors with and without N, heyseana. The average shortage of M. trossulus in collectors with N. heyseana is 805 specimens. The difference between these two values can be explained by the unaccounted-for intervals between attacks. As the size of the M. trossulus increases, the intensity of their consumption decreases. Depending on the time of the year, the daily consumption of M. trossulus by one adult of N. heyseana ranged from 0.05 to 0.50 ind./day, and the average consumption intensity was 0.28 ind./day. Consequently, during the year, one individual of adult N. heyseana ate about 102 specimens of M. trossulus in the aquarium. This was her main and easiest prey.
The results obtained show the significant impact of N. heyseana on the population of cultured mollusks. However, this effect is beneficial for M. yessoensis, since in collectors and cages the predator consumes mainly M. trossulus (Figure 5). The presented materials allow us to recommend the joint cultivation of M. yessoensis with N. heyseana.
Marine gastropods are considered to be one of the most successful objects for the study of many general biological problems.12 In the 60s, no significant accumulations of N. heyseana were formed in the Posyeta Bay.3,19,20 The predators reached the maximum density in the southern Kuril Islands - 33-150 ind./m2 with a biomass of 7-90 g/m2.21
The absence of N. heyseana in the shallow bays of the Posyeta Bay is explained by the summer high water temperature (about 30°C), since the southern boundary of the related species N. lapillus is limited by a temperature of 19ºC.1
With an increase in the volume of cultivation in the Minonosok Inlet M. yessoensis and M. trossulus, the abundance of N. heyseana on the bottom increased to 2,200 ind./m2, and the biomass to 6.9 kg/m2.22 The cultivation of M. trossulus contributes to the colonization of coastal communities by its larvae, which increases the fertility of N. heyseana, because an abundance of food provides high energy reserves.23 The number of predators is proportional to the density of the prey settlement and the area of its accumulation,9 and the dynamics of the number of consumers is strictly related to the limiting parameter - food.24
The food presence seems to have an impact and on the vertical distribution of N. heyseana, since in the 60s predators were found from the middle and lower horizons of the littoral and littoral baths to a depth of 1 m. With the appearance of marine plantations in the Posyeta Bay N. heyseana began to occur in scallop collectors placed in the open Raid Pallada bay (Posyeta Bay) to a depth of 17 m (present message).
The calculations using the formula of25 showed that at 15ºC M. trossulus larvae are in plankton for 27 days. A correlation analysis conducted with the 38-year dynamics of the population of N. heyseana and the climatic factors have shown that the wind speed in June has a significant positive effect on its reproduction (r = 0.476; p = 0.002). During their pelagic development, mainly south-eastern winds blow in the Posyeta Bay and the larvae can be carried away from the Minonosok Inlet over long distances. In this case, the larvae of N. heyseana, which have been in the plankton for about 4 hours, could have lost food by the time they reached metamorphosis, if not for the mechanism of their return to their parents.26 Most of the late larvae have negative phototaxis and descend into the bottom horizons,27 migrating with the bottom stream to the shore28 and there the predator and victim find each other.
The larvae of the planktotrophic M. trossulus have a negative impact on the community of small phytoflagellates in the Vostok Bay,29 which is typical for planktophages30 and M. trossulus begins to spawn earlier than A. amurensis, which has a planktotrophic larva.31 The area of plantations in Minonosok Inlet is not less than in Vostok Bay, therefore, the early finding of M. trossulus larvae in plankton and their eating of phytoplankton is probably one of the main factors that reduced the abundance of A. amurensis juveniles in scallop collectors by an order of magnitude in 1979 - 1989.32
During the period of high trophic load on diatoms caused by the joint cultivation of M. yessoensis and M. trossulus in the Minonosok Inlet, the abundance of A. amurensis juveniles in collectors decreased, and N. heyseana, which has a stock of yolk and does not need phytoplankton, increased (Figure 4). The ability to feed on older victims allows N. heyseana to lag behind by a year the dynamics of their main food abundance, M. trossulus, and the dynamics of victim-predator number acquires a classic appearance.33-35 However, the juveniles of A. amurensis cannot overpower one-year-old M. yessoensis, so their population dynamics coincide.36
In A. amurensis larvae, a preferred colonization of collectors with a larger number of mollusks was found,37 according to literature data, chemotaxis is well known in gastropods when choosing a substrate by larvae.13 Probably, it is also available in N. heyseana, since the abundance of M. yessoensis in collectors with N. heyseana was higher than without it (on average 856 and 796 specimens accordingly).
outside,38,39 many publications have noted the desire of predators to optimize the nutrition by selecting the largest victims5,6,40,41 and the rate of eating depends on the size of the predator.42 At the same time, the size selection can lead to depletion of the main age class.43,44 However, by eating large individuals, N. heyseana and A. amurensis reduce the total number of M. trossulus and its growth rate increases (Figure 8) and the survival rate of the surviving individuals. 22 The importance of predation decreases with the thickening of the shell45 and an increase in the size of the victim.46 Having reached a height of 40 mm, M. trossulus becomes invulnerable,41 however, N. heyseana is a more dangerous predator, eating 59 mm individuals.
The intensity of Nycella predation largely depends on temperature and increases before spawning,40,23,47 which is confirmed by our aquarium observations. In winter, the nutritional requirement of one individual of N. heyseana did not exceed 0.05 specimens of M. trossulus per day, and before spawning it increased to 0.50 specimens per day (Figure 14). Due to global warming, the predatory activity of N. heyseana may increase with increased energy exchange.47
In predatory gastropods, the assimilation efficiency is higher than in herbivores and it ranges from 60 to 90%,13 and in N. lapillus it reaches 66%.40 We obtained similar values for N. heyseana on artificial substrates and in an aquarium (63.6 and 65.5%, respectively).
The effectiveness of gastropods as predators depends on the surf strength of waves and drainage.48-50 Perhaps, therefore, the growth rate and the life duration N. heyseana in collectors exceed those living on the bottom, 22 and the compensatory growth observed in many animals51 is also observed in N. heyseana in the aquarium (Figure 11). The larvae of cultured mollusks settling to the bottom increase the abundance of food for N. heyseana there and they reach 44 mm in shell height, and the largest individual of N. heyseana in the 60s reached 38 mm at the bottom.3 The fertility of N. heyseana has also increased, as their abundance at the bottom has increased (present message).
The ability of large N. lapillus individuals to push apart the flaps of Mytilus edulis9 and even use drugs41 somewhat conceal their predatory role, since there are no holes on the empty flaps. Perhaps, therefore, the observed preference of the large N. heyseana for the small M. trossulus (Figure 9) arose from the fact that the part of the large M. trossulus (13%) fell into the group of deaths due to natural causes. During the feeding process, predators can switch from their favorite food to an energetically more profitable one, and the preferred species has a chance to preserve the population.2,10 However, the littoral M. trossulus are doomed to complete destruction due to the hydrology of the area.6,7 According to observations in Troitsa Inlet, by the end of June, we found only 50 individuals of M. trossulus of spawning two-year age. The cape where they survived was characterised by strong surf.
In littoral biocenosis, the mussels are often a competitive dominant and they are the preferred food of starfish,52 Tais and Nucella8,48 increases the species diversity of the community. A slight increase in the number of bivalves species with N. heyseana in scallop collectors was also detected in our experiments. In part, this smooths out the negative impact of mariculture plantations on the species richness of benthic communities.53
The Global warming has a positive effect on the reproduction of M. trossulus, since we found in the Posyeta Bay a positive correlation coefficient (r = 0.2354; p = 0.155) between the 38-year-old dynamics of water temperature in June and the abundance of its juveniles in scallop collectors. As a result of an increase in the abundance of food, the fertility of N. heyseana will increase. N. heyseana and its juveniles will begin to populate scallop collectors and cages again, which will have a positive effect on the production performance of M. yessoensis and the surviving of M. trossulus. By the end of the fifth month of life of M. yessoensis in collectors, their survival and size were higher with N. heyseana.22 In the case of N. heyseana settling into cages with one-year-old scallops, the predator, having eaten 102 specimens of M. trossulus in a year, can down two-year-old M. yessoensis (shell height 73 3 mm). However, the survival rate of the remaining M. yessoensis, their size, weight of soft tissues and the muscle turned out to be higher than that of those living in a cage with two A. amurensis and especially without predators.22
The publications on the absence of a pelagic larva in the genus Nucella (Thais) contradict the data of28 on the pelagic larva in T. haemastoma in the tropical Atlantic Ocean and in T. emarginata at the California coast. The presence of a pelagic larvae in N. heyseana, which lives on artificial substrates, as well as the presence of other gastropods on such substrates: Boreotrophon candelabrum and Mitrella burchardi suggest a pelagic stage in them. At least M. lunata has it55 cited by.54
The results of our experiments showed the negative impact of the Pacific mussel M. trossulus on the production parameters of cultivated M. yessoensis and the preferred consumption of M. trossulus by a predator – N. heyseana allows us to recommend it as a biological defense against competitors of M. yessoensis (Figures 15&16).
We express our sincere gratitude to N.A. Aizdaicher, an employee of the National Scientific Center for Marine Biology of the Far Eastern Branch of the Russian Academy of Sciences, for providing a mixture of microalgae used for feeding M. trossulus, and to I.D. Rostov, an employee of the Pacific Oceanological Institute, for climatic data collected by the hydrometeorological station of the Posyet village.

©2025 Gabaev, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.