Journal of
eISSN: 2373-6437


Review Article Volume 17 Issue 2
1Obstetric Intensive Care Medicine, Autonomous University of the State of Mexico, Mexico
2Clinical Nutritrion, Marist University of Mérida A.C., Mexico
3Investigation, National Polytechnic Institute, Mexico
4Clinical Nutritrion, La Salle University, Mexico
5Intensive Care Medicine, National Autonomous University of Mexico, Mexico
6Obstetric Intensive Care Medicine, Army and Air Force University, Mexico
7Gynecologic Oncology, National Autonomous University of Mexico, Mexico
8Hematology and B. Marrow Transplantation, National Autonomous University of Mexico, Mexico
Correspondence: Jesser M. Herrera-Salgado, Obstetric Intensive Care Medicine, Autonomous University of the State of Mexico, Mexico, Tel +525581399044
Received: March 25, 2025 | Published: April 23, 2025
Citation: Herrera-Salgado JM, Solís-Loría WA, Solís-Loría PV, Dueñas-Sosa M, Rivas-Rivas FR, Herrera-Villalobos JE et al. Refeeding Syndrome: Review Article. J Anesth Crit Care Open Acces. 2025;17(2):62-68. DOI: 10.15406/jaccao.2025.17.00621
Refeeding syndrome is a potentially life-threatening condition that occurs when nutritional support is reintroduced after prolonged starvation or malnutrition. It primarily affects high-risk groups, such as individuals with eating disorders and patients with renal failure undergoing hemodialysis. This syndrome is marked by profound metabolic and electrolyte disturbances, including hypophosphatemia, hypokalemia, hypomagnesemia, and thiamine deficiency, which can lead to severe cardiovascular, hematologic, and neurological complications. A literature review was conducted using key words: "Refeeding syndrome," "Malnutrition," "Electrolyte imbalance," and "Critical care nutrition." This process identified 94 PUBMED articles, of which 31 were selected based on their relevance to the pathophysiology, prevention, diagnosis, and treatment strategies of the condition.
Diagnosing refeeding syndrome is particularly challenging, as it necessitates ruling out other acute conditions, such as fluid overload, which can significantly lower plasma electrolyte levels. Moreover, the syndrome's aftermath requires thorough assessment, especially in cases involving independent cardiac arrhythmias like long QT syndrome, which pose heightened risks due to electrolyte imbalances. Prevention strategies focus on the early identification of at-risk patients, a controlled and gradual increase in caloric intake, and stringent electrolyte monitoring. Although various management approaches have been proposed, significant debate continues due to the lack of standardized data and quantification methods. This article offers an updated review, compiled by a team of experts, integrating the latest scientific findings from medical literature to deepen understanding and improve clinical management of refeeding syndrome.
Keywords: Refeeding syndrome, Malnutrition, Electrolyte imbalance, Critical care nutrition
A comprehensive literature review was conducted using the keywords: Refeeding Syndrome, Malnutrition, Electrolyte Imbalance, and Critical Care Nutrition. The search encompassed studies published within the last two decades, utilizing the PubMed database. An initial screening identified 94 articles matching the specified keywords. Subsequently, a selection process was applied, refining the dataset to 31 manuscripts that specifically addressed the key aspects relevant to this review. The final selection was based on their direct relation to the core topics under investigation.
Critically ill patients often face persistent low-grade inflammation, suppressed immunity, and ongoing catabolism despite nutritional interventions. Despite clinical improvement within 3 to 7 days in the ICU, some patients develop Persistent Inflammatory Catabolism Syndrome (PICS), characterized by ongoing inflammation and protein catabolism.1,2 Critically ill patients undergo significant decomposition of macronutrients, particularly evident in skeletal muscle proteolysis. Persistent inflammation in PICS contributes to skeletal muscle wasting due to increased protein degradation and decreased synthesis.3,4 ESPEN (2019) recommends 1.3 g/kg of protein daily for critically ill patients. Current therapy for PICS includes high protein intake (1.5 to 2.0 grams/kg) and leucine supplementation, along with anabolic agents and fish oil-derived mediators. Combining these interventions with physical therapy and resistance exercise training is crucial. Refeeding syndrome (RFS) arises from fluid and electrolyte shifts following aggressive nutritional rehabilitation after prolonged starvation. It leads to complications like decreased serum phosphate, potassium, and magnesium levels, potentially causing organ damage. Malnourished patients resuming feeding after significant caloric restriction are at risk of RFS, which can occur with any form of feeding. The American Society for Parenteral and Enteral Nutrition (ASPEN) proposes standardized diagnostic criteria based on severity levels.5-7
This manuscript presents a comprehensive review conducted by a team of experts, focusing on the key elements essential for understanding the pathophysiology of Refeeding Syndrome, as well as its prevention, diagnosis, and treatment. The analysis integrates current evidence and clinical perspectives to provide a structured approach to managing this condition.
Pathophysiology of Refeeding syndrome
Patients with severe trauma, infections, or shock states exhibit elevated hepatic glucose production and muscle protein catabolism. Prolonged fasting or starvation exacerbates metabolic disorders and increases the risk of refeeding syndrome upon abrupt nutritional therapy initiation. This can lead to multiple organ failure within the first 72 hours. Malnutrition is a significant risk factor, with definitions proposed by ASPEN and the European Society for Clinical Nutrition and Metabolism (ESPEN) based on malnutrition etiology. In Table 1, the classification of malnutrition is explained according to ASPEN and ESPEN.6-8 During malnutrition, the body stays in hypometabolism to preserve metabolic homeostasis and nutrients. This occurs due to increased counterregulatory hormones like growth hormone (GH), glucagon, cortisol, catecholamines, and decreased triiodothyronine, IGF-1, insulin, and leptin. Reduced sympathetic nervous system activity also leads to a decrease in basal cerebral metabolism.
|
Etiology |
Characteristics |
|
Malnutrition secondary to starvation |
Chronic absence of food intake, without an inflammatory process, leads to depletion of the body's fat reserves, typically observed in eating disorders. |
|
Malnutrition secondary to chronic illness |
There is chronic mild-to-moderate inflammation with degradation of lean body mass secondary to the inflammatory process. |
|
Malnutrition secondary to acute illness |
There is an acute inflammatory process combined with a caloric-protein deficit; this type of malnutrition requires intensive medical and nutritional management. |
Table 1 Etiological classification of malnutrition according to ASPEN and ESPEN
Fasting phases
First phase: Glucose from circulating levels and glycogen reserves in muscle and liver is used. Hypoglycemia occurs after 24 hours.
Second phase: Lipid consumption starts around 48 hours into fasting to maintain energy balance. The body uses ketone bodies from lipolysis while decreasing protein catabolism. Metabolic acidosis follows due to fixed organic fatty acids compared to carbonic acid. Compensatory mechanisms include CO2 elimination and renal excretion of hydrogen ions, phosphate, and ammonium.
Third phase: Essential proteins are consumed as reserves deplete, increasing mortality risk.
Critically ill patients often experience hyperinsulinism when refeeding begins, leading to increased energy expenditure and potential complications like hyperlactatemia and metabolic acidosis due to low thiamine levels. Hyperinsulinemia also causes intracellular ion influx, sodium retention, and edema.8-10
Phosphorus depletion affects energy genesis, causing issues in glycolytic enzyme activity and leading to symptoms below 1.5 mg/dL. Potassium and magnesium ion imbalances result in muscular, gastrointestinal, and cardiac disturbances.11,12
Organic alterations
Hypophosphatemia
Nervous system: Tissue hypoxia causes neurological symptoms ranging from fatigue to paralysis.
Cardiovascular system: This leads to myocardial atrophy, impaired contractility, and volume overload, resulting in heart failure and arrhythmias.
Respiratory system: Respiratory failure due to reduced muscle contractility and altered vital capacity.
Hematological system: Causes hemolytic anemia, thrombocytopenia, and sepsis risk.
Musculoskeletal system: Muscle weakness, myalgias, and rhabdomyolysis are common.
Refeeding syndrome can also increase CO2 production, leading to respiratory acidosis and neutrophil function impairment. Monitoring and addressing nutrient deficiencies are crucial for preventing complications. 8-13
Hypomagnesemia
Magnesium is the second most abundant intracellular cation, predominantly found within bone and muscle. Consequently, serum levels do not accurately reflect the body's total magnesium content. Only 30% of ingested magnesium is absorbed in the proximal small intestine; the remaining 70% is excreted through feces, with renal excretion being predominant. Magnesium functions at the cellular level as a cofactor for numerous enzymes involved in various biochemical reactions, such as oxidative phosphorylation. Normal serum levels range between 1.8–2.5 mg/dL. Patients with mild to moderate hypomagnesemia (serum magnesium levels of 1–1.5 mg/dL) typically do not exhibit symptoms, while those with severe hypomagnesemia (serum magnesium levels <1 mg/dL) may experience diverse clinical manifestations including neuromuscular dysfunction, electrocardiographic abnormalities, cardiac arrhythmias, and even death.
Additionally, hypomagnesemia can induce hypocalcemia due to impaired secretion and action of parathyroid hormone (PTH) on target cells in bone and kidney, as well as vitamin D resistance caused by an abnormality in the 1-alpha-hydroxylation of 25(OH)D in the kidney. Hypokalemia may also occur secondary to disrupted Na+/K+-ATPase activity, leading to increased renal losses of potassium.11-14
Hypokalemia
Potassium is the principal intracellular monovalent cation, with 98% of total body potassium located intracellularly. Its excretion occurs approximately 80% through the kidneys, and the remainder through feces and sweat. Potassium is vital for various physiological functions, including maintaining membrane potential and regulating glycogen and protein synthesis. Therefore, hypokalemia disrupts transmembrane action potentials, resulting in hyperpolarization and altered muscle contractility.12-16
In mild to moderate hypokalemia (2.5–3.5 mEq/L), gastrointestinal symptoms such as nausea, vomiting, and constipation may occur. If untreated, severe hypokalemia (serum potassium <2.5 mEq/L) may present with neuromuscular dysfunction, disturbances in myocardial contractility, and changes in electrocardiogram such as ST segment depression, flattening or inversion of T waves, or the presence of U waves. There is also an increased risk of cardiac arrhythmias, ranging from atrial tachycardia and bradycardia to ventricular tachycardia and fibrillation, potentially leading to sudden death.13-16
Other clinical manifestations include glucose intolerance, metabolic alkalosis, worsening hepatic encephalopathy, and increased digitalis toxicity.
Thiamine deficiency
Thiamine, or vitamin B1, is a water-soluble vitamin essential for carbohydrate metabolism, acting as a cofactor for pyruvate dehydrogenase and transketolases. The body's thiamine reserves are approximately 30 mg, and its short half-life (9.5–18.5 days) means rapid depletion occurs in cases of malnutrition. Over 80% of thiamine is absorbed in the proximal small intestine via active transport. Thiamine deficiency results in elevated serum pyruvate concentration, which is converted into lactate. At-risk groups include chronic alcoholics, individuals with malabsorption syndromes, and those experiencing nausea and vomiting during pregnancy. Thiamine deficiency can lead to heart failure, Wernicke encephalopathy, and Korsakoff syndrome.14-17
Sodium retention/overload
Carbohydrate intake during the initial phases of refeeding syndrome decreases renal sodium excretion and expands extracellular fluid, increasing the risk of cardiac decompensation. This risk is heightened in patients with severe malnutrition due to potential atrophy and reduced contractility.16-19 In Figure 1, the cellular phenomena involved in Refeeding Syndrome are explained in detail. In Table 2, a summary of the clinical organic alterations secondary to hydroelectrolytic imbalance is presented.
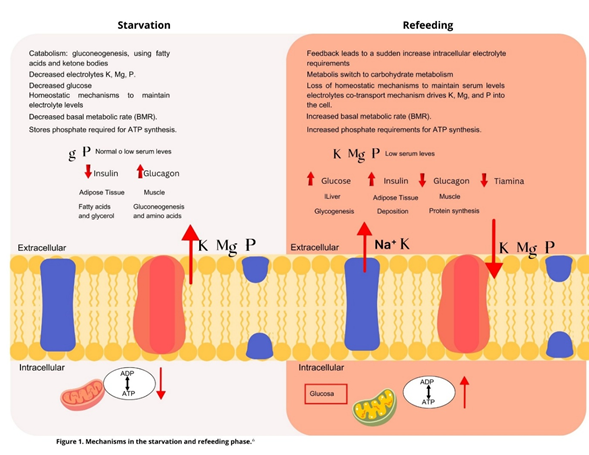
Figure 1 Molecular Pathophysiological Mechanisms in Refeeding Syndrome. Pathophysiological Mechanisms in Refeeding Syndrome (Figure 1. Modified from Marino et al., 2007 by Dr. Rosa Esther Moreno López. .
|
Organic Alterations |
Serum Electrolytes |
||||
|
Hypophosphatemia |
Hypokalemia |
Hypomagnesemia |
Thiamine Deficiency |
Sodium Retention |
|
|
Neurological |
Paresthesia |
Paresthesia, areflexia, muscle necrosis |
Ataxia |
Peripheral neuritis |
Cerebral edema |
|
Acute areflexic paralysis |
Irritability |
Wernicke encephalopathy |
|||
|
Weakness |
Weakness |
Korsakoff syndrome |
|||
|
Asthenia |
Tremor |
||||
|
Adynamia |
Ataxia |
||||
|
Lethargy |
Nystagmus |
||||
|
Drowsiness |
Vertigo |
||||
|
Confusion |
Tetany |
||||
|
Disorientation |
Hyperreflexia |
||||
|
Seizures |
Personality alterations |
||||
|
Coma |
Irritability |
||||
|
Depression |
|||||
|
Seizures |
|||||
|
Coma |
|||||
|
Cardiac |
Impaired systolic function |
Impairment in cardiac conduction and contractility |
Electrocardiographic changes (PR interval prolongation, QRS widening, QT prolongation, ST depression, peaked or flattened T waves), arrhythmias (atrial fibrillation, ventricular arrhythmias, torsade de pointes, ventricular extrasystoles) |
Heart failure |
Heart failure |
|
Congestive heart failure |
Electrocardiographic abnormalities (ST segment depression, T wave flattening or inversion, presence of U waves) |
||||
|
Hypotension |
Arrhythmias (atrial tachycardias, bradycardias, atrioventricular blocks, ventricular tachycardia or fibrillation) |
||||
|
Pericardial effusion |
Increased sensitivity to digitalis toxicity |
||||
|
Cardiac arrhythmias |
Cardiac arrest |
||||
|
Cardiogenic shock |
Sudden death |
||||
|
Sudden death |
|||||
|
Respiratory |
Dyspnea |
Impairment in respiratory function |
---- |
---- |
Acute pulmonary edema |
|
Impaired diaphragmatic contractility |
Respiratory failure due to diaphragmatic paralysis |
||||
|
Acute respiratory distress syndrome |
|||||
|
Difficult ventilatory weaning |
|||||
|
Gastrointestinal |
Anorexia |
Nausea |
Anorexia |
--- |
|
|
Nausea |
Vomiting |
Nausea |
|||
|
Vomiting |
Ileus |
Vomiting |
|||
|
Constipation |
Diarrhea |
||||
|
Worsening of hepatic encephalopathy |
|||||
|
Metabolic |
Hypomagnesemia |
Carbohydrate intolerance, metabolic alkalosis |
Refractory hypocalcemia |
Lactic Acidosis |
|
|
Hypokalemia |
|||||
|
Hematological |
Hemolytic anemia |
--- |
----- |
--- |
|
|
Thrombocytopenia |
|||||
|
Alterations in platelet and leukocyte function |
|||||
|
Kidney |
Acute tubular necrosis |
Polyuria |
----- |
--- |
|
|
Skeletal muscle |
Weakness |
Weakness |
Weakness |
--- |
|
|
Myalgias |
Rhabdomyolysis |
Fasciculations |
|||
|
Rhabdomyolysis |
Muscle dysfunction |
Tetany |
|||
|
Diaphragmatic weakness |
Muscle necrosis |
||||
|
Proximal myopathy |
|||||
Table 2 Refeeding Syndrome by Organ Systems
*Significant (P≤0.05); NSNot significant (P>0.05)
Pathophysiological mechanisms in Refeeding syndrome
Refeeding syndrome is an anabolic state triggered by nutritional therapy, accompanied by hydroelectrolytic imbalances such as hypophosphatemia, hypocalcemia, hypomagnesemia, hyperglycemia, and thiamine deficiency. Hypophosphatemia, unique to this syndrome, affects enzymatic systems and ATP production, leading to energy deficits, apoptosis, and increased risk of hypoxia due to impaired oxygen release from hemoglobin. During starvation or critical illness, insulin secretion decreases while glucagon increases, promoting gluconeogenesis and depleting glycogen and amino acid reserves, eventually shifting to ketone-based metabolism. This reduces basal metabolic rate and disrupts sodium/potassium ATP pump activity, causing intracellular cation leakage. With renewed nutritional intake, glucose and insulin rise, leading to intracellular shifts of electrolytes like potassium, phosphate, and magnesium, further depleting their extracellular concentrations. This triggers sodium and water retention, expanding extracellular volume. Increased thiamine demand, essential for enzymatic reactions, cellular respiration, and energy production, exacerbates the condition, underscoring the critical need for careful management of refeeding.
Classification according to the risk of Refeeding syndrome
Evaluating the risk of malnutrition is essential to identify patients susceptible to refeeding syndrome. Patients with minimal or no nutritional intake for several consecutive days or experiencing metabolic stress due to critical illness or major surgery are at risk of developing RFS. Screening strategies for identifying patients at high risk of refeeding syndrome are imprecise, and there is a lack of a consensual definition. However, criteria have been published to predict refeeding syndrome, including those developed in 2006 by the National Institute for Health and Care Excellence (NICE) in the United Kingdom. These criteria were formulated based on published reviews and the authors' experience, agreed upon by informal consensus, and stratified into two major groups: "low risk and high risk." It is recommended that any patient with one or more high-risk factors or two or more low-risk factors receive professional medical care with monitoring for complications.16-20 Subsequently, the Irish Society for Clinical Nutrition and Metabolism (IrSPEN) in its refeeding syndrome management guidelines published in 2013 and the expert consensus by Friedli et al., published in 2018, recommended classification into three respective groups: "moderate risk, low risk " and "high risk – very high risk". Table 3 presents the risk stratification according to the following consensuses: NICE, IrSPEN, and Friedli.6-8,18,21-24
|
NICE |
IrSPEN |
Friedli et al. |
|
|
Risk stratification |
Low risk: |
Moderate risk: |
Low risk: |
|
BMI below 18.5 Kg/m2 |
One of the low-risk features of the NICE Guidelines |
One of the low-risk features of the NICE Guidelines |
|
|
Unintentional weight loss of more than 10% in the last 3 to 6 months |
|||
|
Little or no food intake for more than 5 days |
|||
|
History of alcohol or drug abuse; including insulin, chemotherapy, antacids and diuretics |
|||
|
High Risk: |
High risk: |
High risk: |
|
|
BMI below 16 kg/m2 |
Two of the low-risk features of the NICE guidelines |
Two of the low-risk features of the NICE guidelines |
|
|
Unintentional weight loss of more than 15% in the past 3 to 6 months |
One of the high-risk features of the NICE guidelines |
One of the high-risk features of the NICE guidelines |
|
|
Little or no food intake for more than 10 days |
|||
|
Decreased serum levels of phosphorus, potassium, or magnesium before starting feeding |
|||
|
Very high risk |
Very high risk |
||
|
Body mass index less than 14 kg/m2 |
Body mass index less than 14 kg/m2 |
||
|
Little or no food intake for more than 15 days |
Little or no food intake for more than 15 days |
||
|
Weight loss greater than 20% in a period of 3 to 6 months |
Table 3 Risk stratification for refeeding syndrome according to the National Institute for Health and Care Excellence (NICE), Irish Society for Clinical Nutrition and Metabolism, (IrSPEN)
Table 4 presents the consensus criteria for identifying adult patients at risk of RFS according to the ASPEN. These include several additional points to the previously mentioned criteria, consistent with the characteristics of moderate and severe malnutrition.
|
Moderate risk: 2 risk criteria needed |
Significant risk: 1 risk criterion needed |
|
|
Body mass index |
16–18.5 kg/m2 |
< 16 kg/m2 |
|
Weight loss |
5% in 1 month |
7.5% in 3 months or > 10% in 6 months |
|
Caloric intake |
None or negligible oral intake for 5–6 days OR < 75% of estimated energy requirement for > 7 days during an acute illness or injury OR < 75% of estimated energy requirement for > 1 month |
None or negligible oral intake for > 7 days OR < 50% of estimated energy requirement for > 5 days during an acute illness or injury OR < 50% of estimated energy requirement for > 1 month |
|
Prefeeding potassium, phosphate, or magnesium serum concentrations |
Minimally low levels or normal current levels and recent low levels necessitating minimal or single-dose supplementation |
Moderately/significantly low levels or minimally low or normal levels and recent low levels necessitating significant or multiple‐dose supplementation |
|
Loss of subcutaneous fat |
Evidence of moderate loss |
Evidence of severe loss |
|
Loss of muscle mass |
Evidence of mild or moderate loss |
Evidence of severe loss |
|
Higher‐risk comorbidities |
Moderate disease |
Severe disease |
Table 4 ASPEN consensus criteria for identifying adult patients at risk of RFS
Due to the above, it is recommended to conduct malnutrition screening within the first 24-48 hours using tools like NRS-2002, MNA-SF, MUST, and SNAQ.
Diagnostic approach
Most definitions for diagnosing refeeding syndrome have focused on hypophosphatemia. The ASPEN guidelines suggest a unified criterion to classify severity into mild, moderate, and severe. This includes reductions in phosphate, potassium, magnesium, thiamine, and clinical symptoms or organ dysfunction within the first 5 days after starting nutritional support. Any decrease may indicate a total body deficit requiring monitoring and intervention (Table 5).20-24
|
Severity |
Definition |
|
Mild |
A decrease of 1, 2, or 3 serum phosphorus, potassium, or magnesium levels by 10% to 20%, occurring within 5 days of nutritional support. |
|
Moderate |
A decrease of 1, 2, or 3 serum phosphorus, potassium, or magnesium levels by 20% to 30%, occurring within 5 days of nutritional support |
|
Severe |
A decrease of 1, 2, or 3 serum phosphorus, potassium, or magnesium levels by >30% or organ dysfunction resulting from a decrease in any of these or due to thiamine deficiency (severe), occurring within 5 days of nutritional support. |
Table 5 Diagnostic criteria by ASPEN guidelines
Differential diagnosis of Refeeding syndrome
RFS is a diagnosis of exclusion, requiring careful differentiation from other acute conditions.
Hypercapnic acidosis: Related to metabolic changes due to overfeeding and must be differentiated from respiratory failure.
Metabolic alterations secondary to overfeeding: Include hypercapnia and respiratory acidosis, potentially leading to respiratory failure in patients with limited lung reserve.
Fat overload syndrome: Characterized by hyperlipemia, fever, fat infiltration, hepatomegaly, splenomegaly, anemia, leucopenia, thrombocytopenia, and coagulopathy.
Electrolyte imbalances: Hypophosphatemia, hypokalemia, and hypomagnesemia can appear in other nutritional and metabolic disorders.
Severe malnutrition: Shares symptoms with refeeding syndrome but requires a different management approach.18,20,24-26
Treatment and prevention
Summary of the critical clinical considerations to be taken into account in the treatment and prevention of refeeding syndrome. (See Figure 2) To treat and prevent this condition in adults, several key steps should be followed:
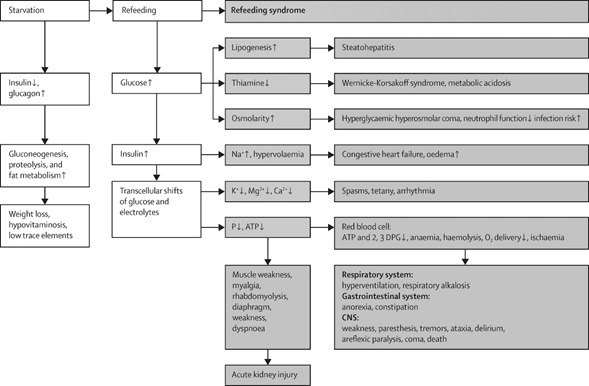
Figure 3 A final summary of critical clinical and biochemical events during prolonged fasting and Refeeding Syndrome.
Modified from.31
Refeeding syndrome poses a significant risk, leading to severe metabolic, cardiovascular, hematologic, gastrointestinal, and neurological complications. To prevent and effectively manage this condition, healthcare professionals—particularly intensive care physicians oncologists and nutritionists—must have a thorough understanding of its pathophysiology and risk factors.
Early identification and meticulous nutritional intervention are essential to minimizing adverse outcomes. Implementing strategies such as gradually increasing caloric intake and closely monitoring electrolyte levels can help mitigate risks and ensure a safer recovery for patients.
None.
None.

©2025 Herrera-Salgado, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.