Journal of
eISSN: 2572-8466


Review Article Volume 12 Issue 1
1Department of Biotechnology and Bioinformatics, California State University, USA
2Department of Bioengineering, University of California, USA
Correspondence: Bill Tawil, Department of Bioengineering, UCLA School of Engineering, 420 Westwood Plaza, Room 5121, Engineering V. P.O. Box: 951600, Los Angeles, CA 90095-1600, USA, Fax (310) 794-5956
Received: February 17, 2025 | Published: March 21, 2025
Citation: Patel T, Bill T. Understanding leukemia and its clinical significance. J Appl Biotechnol Bioeng. 2025;12(1):29-37. DOI: 10.15406/jabb.2025.12.00381
Leukemia is a group of cancers affecting the bone marrow and blood, leading to an overproduction of abnormal white blood cells that impair the body's ability to produce healthy blood cells, causing complications such as anemia, bleeding disorders, and weakened immunity. It is one of the most common cancers in both adults and children, with incidence rates varying by age, sex, and geography. Although the exact causes remain unclear, factors such as genetic mutations, exposure to radiation, certain chemicals, and infections have been linked to its development. Recent advances in the molecular understanding of leukemia have enabled the development of targeted therapies, improving treatment outcomes and survival rates. Leukemia is classified into four main types—ALL, AML, CLL, and CML—each presenting unique challenges in treatment and prognosis, with outcomes influenced by factors like age and genetic mutations. Despite improvements in chemotherapy, targeted therapies, and immunotherapies, significant challenges such as drug resistance, disease relapse, and the aggressive nature of some leukemia types persist. Early diagnosis through blood tests, bone marrow biopsies, and genetic profiling is critical for determining the most effective, personalized treatment plans. Novel therapies, including CAR-T cell therapy and CRISPR-based approaches, are showing promise in overcoming resistance and targeting specific genetic mutations driving the disease. While advances have been substantial, ongoing research is essential to further refine these treatments, improve long-term outcomes, and address the complexities of drug resistance and relapse in high-risk patients.
Keywords: leukemia, molecular mechanisms, genetic mutations, targeted therapy, immunotherapy, chemotherapy, drug resistance, stem cell transplantation, CRISPR technology, CAR T-cell therapy, personalized medicine, philadelphia chromosome, BCR-ABL fusion gene, FLT3 mutation, minimal residual disease, tyrosine kinase inhibitors
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; MRD, minimal residual disease; TKIs, tyrosine kinase inhibitors; CAR-T, chimeric antigen receptor T-cell therapy; CRISPR, clustered regularly interspaced short palindromic repeats; GVHD, graft-versus-host disease; Ph+, philadelphia chromosome positive; CNS, central nervous system; BCR-ABL, breakpoint cluster region-abelson; TP53, tumor protein p53, FLT3, Fms-like tyrosine kinase 3; IDH1/IDH2, isocitrate dehydrogenase 1/2; T-PLL, T-cell prolymphocytic leukemia
Leukemia is a group of cancers that primarily affect the bone marrow and blood, resulting in the overproduction of abnormal white blood cells.1 These malignant cells impair the body’s ability to produce healthy blood cells, leading to a range of complications such as anemia, bleeding disorders, and a weakened immune system.2,3 Leukemia is one of the most common types of cancer in both adults and children, with its incidence varying by age, sex, and geographic region. While the exact cause of leukemia remains unclear, factors such as genetic mutations, exposure to radiation, certain chemicals, and infections have been associated with its development.1,3 Over recent decades, significant progress has been made in understanding the molecular biology of leukemia, enabling the development of more targeted and effective therapies.2
Leukemia can affect people of all ages, but the prognosis and treatment options differ widely depending on the type of leukemia and patient characteristics.2 Children, for example, tend to have better survival outcomes with certain types, such as acute lymphoblastic leukemia, while older adults with acute myeloid leukemia often face poorer prognoses due to the aggressive nature of the disease and limited treatment options for aging patients.4 Advances in chemotherapy, targeted therapy, and immunotherapy have improved survival rates for many forms of leukemia, but significant challenges remain, particularly in addressing drug resistance and disease relapse.3,4
Classification of leukemia
Leukemia is primarily classified based on the type of blood cell affected and the speed at which the disease progresses. It can be broadly divided into four major types:
Acute lymphoblastic leukemia (ALL)
ALL is a fast-growing form of leukemia that originates in immature lymphoid cells, or lymphoblasts, in the bone marrow. It is the most common form of leukemia in children but also affects adults.4 ALL often requires aggressive treatment, including chemotherapy, radiation, and sometimes bone marrow transplantation.4
Acute myeloid leukemia (AML)
AML arises from the myeloid cell line and is characterized by the rapid growth of abnormal myeloblasts, which interfere with the production of normal blood cells.5 AML primarily affects adults and has a poorer prognosis than ALL, especially in older patients. Treatment typically involves intensive chemotherapy, and outcomes are dependent on the presence of certain genetic mutations and the patient's overall health.5
Chronic lymphocytic leukemia (CLL)
CLL is a slow-growing form of leukemia that originates in mature lymphocytes.6 It is most commonly diagnosed in older adults and often progresses slowly over many years. In some cases, treatment may not be needed immediately, with patients being monitored through a "watch-and-wait" approach.6 However, for more aggressive cases, targeted therapies and monoclonal antibodies are used.1
Chronic myeloid leukemia (CML)
CML is characterized by the uncontrolled growth of mature myeloid cells. It is closely associated with the Philadelphia chromosome, a specific genetic mutation that leads to the production of the BCR-ABL protein, which drives the cancer.7 The discovery of tyrosine kinase inhibitors, such as imatinib, revolutionized the treatment of CML, turning it into a manageable chronic condition for most patients.1
In addition to these primary classifications, leukemia can be further categorized based on specific genetic abnormalities, molecular markers, and risk factors, which help guide treatment decisions and prognostication.8 This molecular understanding has led to more personalized and targeted treatment approaches, improving outcomes for many patients.
Subtypes of leukemia: B-cell and T-cell leukemias
Leukemia can be classified based on the type of blood cell involved. Among the most common classifications are B-cell and T-cell leukemias, which are subtypes of lymphoid leukemias. These subtypes originate from lymphocytes, a type of white blood cell, and are further categorized based on whether the affected lymphocytes are B-cells or T-cells.9 Understanding the differences between these subtypes is crucial for diagnosis, treatment, and prognosis.10
B-cell and T-cell leukemias are subtypes of lymphoid leukemias, each arising from different types of lymphocytes, which are crucial components of the immune system.9,11 B-cell leukemias originate from B lymphocytes, which are responsible for producing antibodies to fight infections. The most common forms of B-cell leukemia include B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia.9,10 B-ALL is the most prevalent type of acute leukemia in children, though it can also occur in adults.9 It is characterized by the rapid proliferation of immature B-cells, known as lymphoblasts, in the bone marrow, leading to symptoms such as anemia, recurrent infections, and organ enlargement due to the infiltration of leukemic cell.11 B-ALL is typically treated with intensive chemotherapy, and in high-risk cases, hematopoietic stem cell transplantation. In recent years, targeted therapies like CAR T-cell therapy, which specifically targets CD19 on B-cells, have significantly improved outcomes for patients with relapsed or refractory B-ALL. On the other hand, CLL, the most common leukemia in adults, particularly the elderly, involves the slow accumulation of mature B-cells in the blood, bone marrow, and lymphoid tissues.10,11 Many CLL patients are asymptomatic at diagnosis, with the disease often being detected incidentally during routine blood tests showing elevated lymphocyte counts. As CLL progresses, it may cause symptoms like fatigue, enlarged lymph nodes, and splenomegaly. Treatment for CLL varies depending on the stage of the disease and symptoms, ranging from watchful waiting in early stages to targeted therapies like BTK inhibitors and monoclonal antibodies in more advanced cases (Figure 1).11
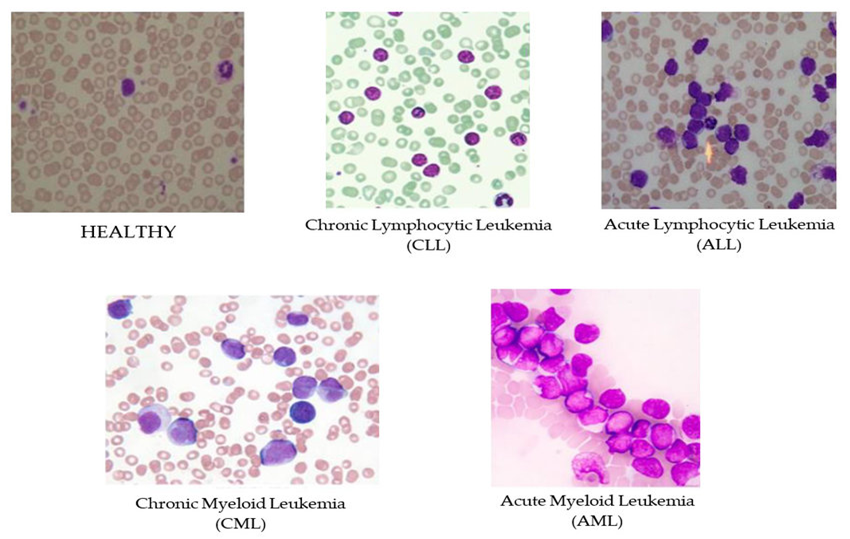
Figure 1 This figure shows the differences in blood cell appearance between a healthy person and individuals with various types of leukemia. The top left image represents a healthy blood smear, where red and white blood cells appear in a normal distribution and shape. The other images illustrate blood affected by different forms of leukemia. In Chronic Lymphocytic Leukemia (CLL), there is an increase in small, mature-looking lymphocytes with slightly irregular shapes. Acute Lymphocytic Leukemia (ALL) shows many large, immature lymphocytes called blasts, which have prominent nuclei. Chronic Myeloid Leukemia (CML) displays an excess of both mature and immature granulocytes, such as neutrophils and basophils. Finally, in Acute Myeloid Leukemia (AML), there is a high concentration of large, immature myeloid cells known as myeloblasts, with noticeable nuclei. These images help doctors identify and understand the specific changes in blood cells that occur with each type of leukemia.47
T-cell leukemias, although less common, tend to be more aggressive compared to B-cell leukemias. These leukemias originate from T lymphocytes, which play a vital role in cell-mediated immunity, including directly killing infected or cancerous cells.9,12 T-cell acute lymphoblastic leukemia is a type of leukemia that is more common in adolescents and young adults. It is characterized by the rapid proliferation of immature T-cells, which can lead to a high white blood cell count and the formation of a mediastinal mass, causing symptoms such as shortness of breath and swelling of the face and arms due to superior vena cava syndrome.11,12 T-ALL is often more aggressive than B-ALL and requires intensive treatment, including chemotherapy and prophylactic or therapeutic CNS-directed therapy, given the higher risk of central nervous system involvement. T-cell prolymphocytic leukemia is a rare but highly aggressive form of leukemia that typically affects older adults. It is characterized by the rapid proliferation of mature T-cells and is often associated with poor prognosis due to its resistance to conventional therapies.9 Patients with T-PLL may present with symptoms such as rapidly worsening lymphocytosis, hepatosplenomegaly, skin involvement, and pleural effusion.11 Treatment options for T-PLL are limited, with monoclonal antibodies like alemtuzumab providing temporary remission and allogeneic stem cell transplantation being the best option for long-term remission in eligible patients. Due to the aggressive nature of T-PLL, the overall prognosis remains poor, with median survival often less than a year despite treatment. Understanding the distinct characteristics and treatment challenges of B-cell and T-cell leukemias is essential for developing effective management strategies and improving patient outcomes (Figure 2).9,10
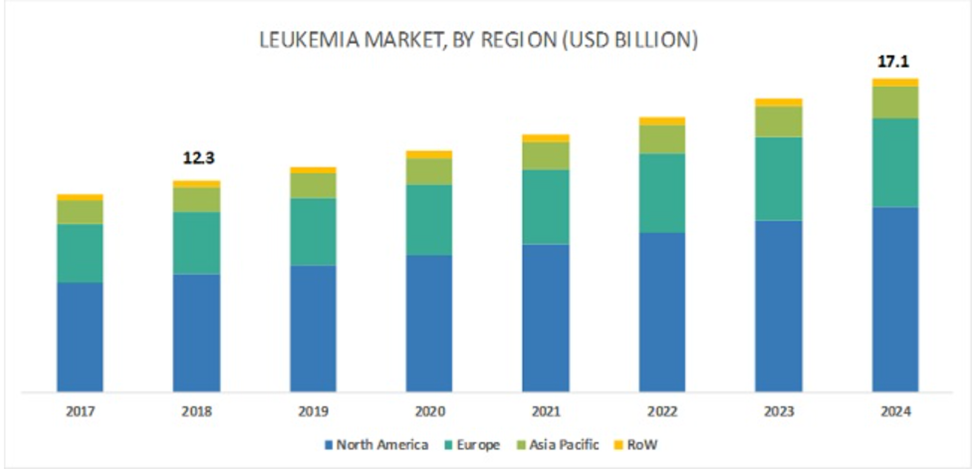
Figure 2 Leukemia Market by Region (2017–2024, USD Billion). This bar chart illustrates the leukemia market size from 2017 to 2024, segmented by geographic regions: North America, Europe, Asia Pacific, and Rest of the World (RoW). The data highlights the growth in market value over the years, with North America consistently holding the largest market share, followed by Europe and Asia Pacific. The market value, which stood at USD 12.3 billion in 2018, is projected to reach USD 17.1 billion by 2024. The increasing market size reflects advancements in leukemia treatments, higher diagnosis rates, and a growing demand for effective therapies across regions.48
Pathophysiology and molecular mechanisms
Leukemia arises from genetic mutations and chromosomal abnormalities that disrupt normal cell processes, leading to the uncontrolled proliferation of abnormal blood cells.13 A key feature of leukemia is the presence of specific genetic alterations, such as mutations in the FLT3, NPM1, and CEBPA genes in acute myeloid leukemia, which influence disease progression and treatment response.14,15 One of the most notable chromosomal abnormalities is the Philadelphia chromosome, a translocation between chromosomes 9 and 22, which creates the BCR-ABL fusion gene.13 This fusion gene produces a protein with continuous tyrosine kinase activity, driving the development of chronic myeloid leukemia and certain cases of acute lymphoblastic leukemia.14,16
At the cellular level, leukemic cells typically fail to differentiate into mature blood cells, resulting in an accumulation of immature precursors, or blasts, in the bone marrow.13,15 This disrupts normal blood cell production, leading to common clinical manifestations such as anemia, infections, and bleeding disorders.13,14 The malignant cells also often develop resistance to apoptosis, contributing to disease persistence and progression. Understanding these molecular and cellular mechanisms has been critical in developing targeted therapies, such as tyrosine kinase inhibitors for CML, which specifically inhibit the abnormal proteins driving leukemia (Figure 3).13,15
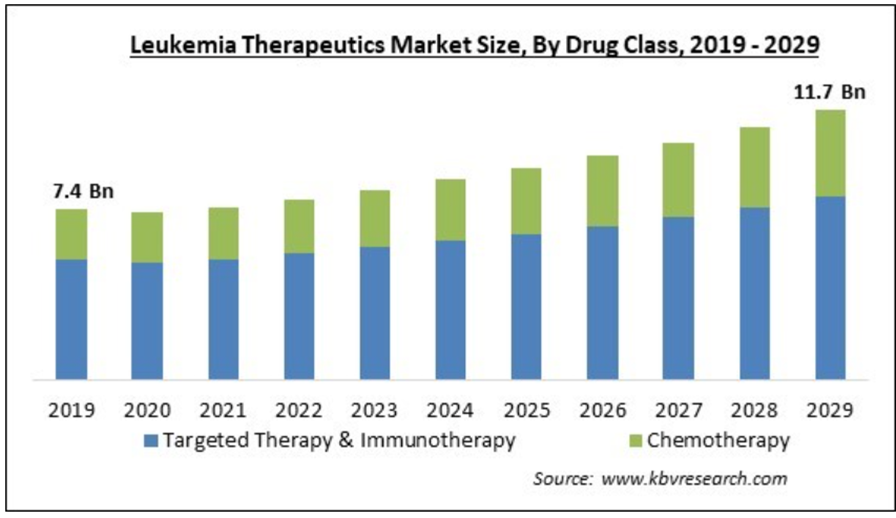
Figure 3 Leukemia Therapeutics Market Size by Drug Class (2019–2029, USD Billion)
This bar chart displays the leukemia therapeutics market size from 2019 to 2029, categorized by drug class: Targeted Therapy & Immunotherapy and Chemotherapy. The chart shows a gradual increase in market size over the years, with an initial value of USD 7.4 billion in 2019 and an expected value of USD 11.7 billion by 2029. Targeted Therapy & Immunotherapy holds a significant and growing portion of the market compared to traditional chemotherapy, reflecting a shift towards more advanced and less toxic treatment options. This trend highlights the rising demand for innovative therapies that offer improved outcomes and reduced side effects for leukemia patients.49
Market size, products, and companies
The global market for leukemia treatments has been growing quickly due to advances in medical research and a rise in the number of people diagnosed with leukemia. This growth is driven by the need for better treatments and improved patient outcomes. In 2023, the global leukemia therapeutics market was valued at around USD 15.25 billion and is expected to grow to over USD 30 billion by 2033. This growth is fueled by the development of new drugs and treatment methods that offer better survival rates and fewer side effects than traditional chemotherapy Acute Myeloid Leukemia, which is one of the most aggressive types, made up a significant portion of the market, valued at USD 2.1 billion in 2023, with an expected growth rate of over 10%. Acute Lymphocytic Leukemia also showed steady growth, valued at around USD 3.12 billion in 2023, as efforts continue to improve treatments with fewer side effects.17
Leukemia treatment has expanded beyond traditional chemotherapy, with new methods that provide more targeted, effective, and less toxic solutions.18 Targeted therapies, such as Imatinib (Gleevec), specifically inhibit proteins that help cancer cells grow, reducing damage to normal cells and improving patients' quality of life.19 Other targeted drugs include Dasatinib (Sprycel) and Nilotinib (Tasigna).18 Immunotherapies, such as Blinatumomab (Blincyto) and Inotuzumab Ozogamicin (Besponsa), help the immune system target leukemia cells, making treatments more effective and minimizing side effects.20 CAR T-cell therapies, like Tisagenlecleucel (Kymriah), involve modifying a patient’s own T-cells to better recognize and kill leukemia cells. This type of personalized treatment is promising, especially for patients who have not responded to other therapies (Figure 4).21
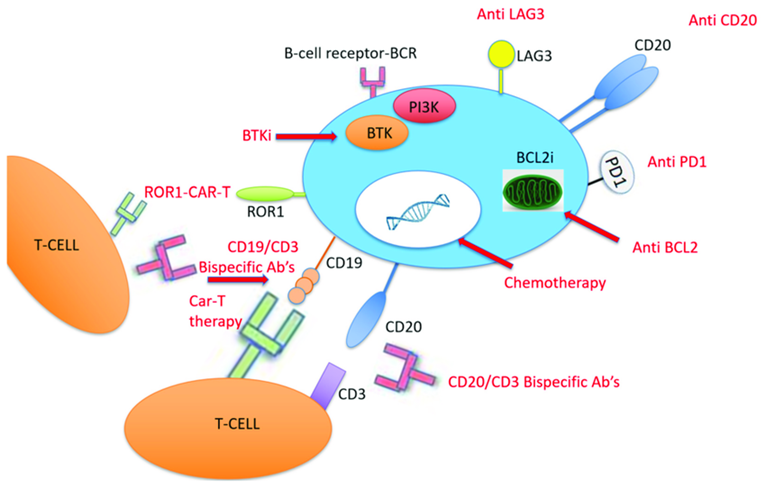
Figure 4 This diagram shows different therapies used to treat Richter Transformation, a condition where a slow-growing leukemia (chronic lymphocytic leukemia, or CLL) becomes more aggressive. In the center is a B-cell, the type of cell affected by CLL. Various treatments target specific parts of this cell to stop it from growing or to help the immune system destroy it. For example, some drugs block the B-cell’s receptors, stopping survival signals. Other treatments, like CAR-T therapy, use T-cells (another type of immune cell) modified to attack the cancerous B-cells by targeting markers like CD19, CD20, and ROR1 found on their surface. Inhibitors like BTK and PI3K also block signals that the cancer cells use to grow. Additional treatments aim at proteins, like BCL2, to make cancer cells more responsive to chemotherapy. Immune checkpoint inhibitors, like Anti-PD1 and Anti-LAG3, help T-cells recognize and attack the cancer cells. Together, these therapies aim to improve the treatment’s effectiveness by focusing on specific parts of the cancer cells and boosting the body’s immune response.50
Several major pharmaceutical and biotech companies are leading leukemia research and treatment development. Bristol Myers Squibb is known for its innovative cancer treatments, including Breyanzi, a CAR T-cell therapy. Novartis has played a major role with treatments like Gleevec and the more recent Scemblix, both of which have improved outcomes for chronic myeloid leukemia patients. Amgen is another significant company, contributing with its development of biologic medicines that target leukemia. Gilead Sciences has invested in developing new drugs for blood cancers and has expanded its capabilities through research partnerships and acquisitions. Sanofi S.A. is also focused on comprehensive leukemia treatments, combining standard approaches with new methods to enhance effectiveness and reduce side effects (Figure 5).22
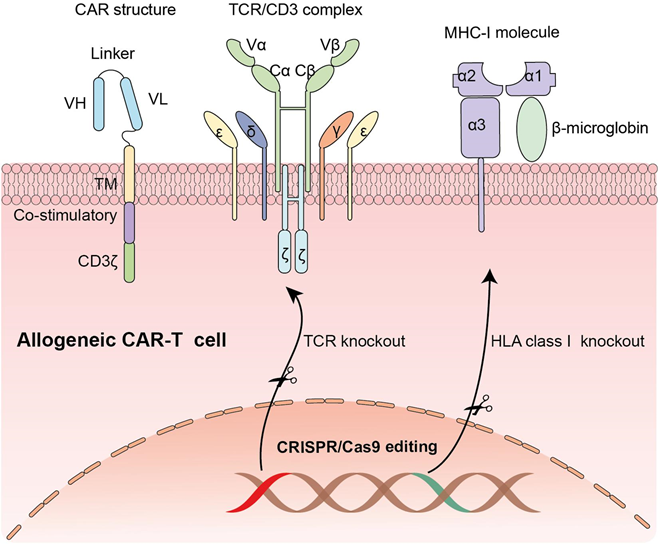
Figure 5 This diagram shows the structure and genetic modification of allogeneic CAR-T cells, which are T-cells modified to attack cancer cells and can be used for different patients. The CAR structure includes parts that allow the T-cell to recognize and attack cancer cells. It has variable regions (VH and VL) that help bind to cancer cells, a signaling part (CD3ζ) to activate the T-cell, and other domains for stability and activation. In normal T-cells, the TCR/CD3 complex helps recognize threats, but in CAR-T cells, the CAR structure takes over this role, allowing direct targeting of cancer cells. To make allogeneic CAR-T cells safe for use in multiple patients, scientists use CRISPR/Cas9 gene editing to "knock out" or remove certain genes. They delete the TCR gene to stop the CAR-T cell from attacking the patient’s healthy cells. They also remove the HLA class I gene to prevent the patient’s immune system from rejecting the CAR-T cells. These genetic changes make allogeneic CAR-T cells safer and more suitable for broader use in cancer treatment.51
Epidemiology of leukemia
Leukemia is a relatively common form of cancer, with its incidence and prevalence varying significantly across different age groups, geographic regions, and populations.23 Globally, leukemia accounts for approximately 2.5% of all cancers, with an estimated 437,000 new cases diagnosed each year.24 The disease is more prevalent in developed countries, with higher incidence rates observed in North America, Europe, and Australia, compared to lower rates in Africa and Asia.
The distribution of leukemia subtypes also varies by age. Acute lymphoblastic leukemia is the most common cancer in children, particularly affecting those under the age of 15, while acute myeloid leukemia and chronic lymphocytic leukemia are more commonly diagnosed in adults, especially those over the age of 60.23–25 Chronic myeloid leukemia is less common but can occur in both younger and older adults.
In terms of gender, leukemia tends to be slightly more common in males than females across most age groups and subtypes.24 Additionally, certain populations, such as individuals with genetic predispositions (e.g., Down syndrome) or those exposed to high levels of radiation or certain chemicals, are at a higher risk of developing leukemia.26
Understanding the epidemiological trends of leukemia is crucial for guiding research, improving early detection strategies, and optimizing treatment protocols tailored to specific populations and risk groups.26
Diagnosis of leukemia
The diagnosis of leukemia typically begins with a thorough clinical evaluation, including a review of the patient's medical history and a physical examination.27 The presenting symptoms often include fatigue, fever, frequent infections, bruising, and unexplained bleeding, which are linked to the bone marrow's failure to produce normal blood cells.27,28 However, since these symptoms can be non-specific, a series of laboratory tests are crucial to confirm the diagnosis.29
Initial blood tests
A complete blood count is the first step in diagnosing leukemia.27 This test measures the number of red blood cells, white blood cells, and platelets in the blood.29 Leukemia is often suspected if there are abnormally high levels of white blood cells or low level of red blood cells and platelets.30 In acute forms of leukemia, immature white blood cells, called blasts, are commonly found in the bloodstream.28
Bone marrow aspiration and biopsy
To confirm a leukemia diagnosis, a bone marrow aspiration and biopsy are typically performed.31 This involves extracting a small amount of bone marrow, usually from the hip bone, to examine the cellular composition.27 Under the microscope, the presence of abnormal leukemic cells in the bone marrow confirms the diagnosis.31 The proportion of blasts in the bone marrow helps differentiate between acute and chronic forms of leukemia, with acute leukemia often showing a higher percentage of these immature cells.29
Cytogenetic and molecular testing
Cytogenetic and molecular testing play a crucial role in diagnosing and classifying leukemia.29 Cytogenetic analysis detects chromosomal abnormalities such as translocations or deletions. For example, the Philadelphia chromosome, a translocation between chromosomes 9 and 22, is a hallmark of chronic myeloid leukemia and can also be found in some cases of acute lymphoblastic leukemia.27
In addition, molecular tests like polymerase chain reaction fluorescence in situ hybridization are used to detect specific genetic mutations or chromosomal abnormalities. These tests can identify mutations in genes such as FLT3, NPM1, and TP53, which help classify the subtype of leukemia and guide treatment decisions.31
Flow cytometry
Flow cytometry is a crucial tool in leukemia diagnosis, providing detailed insights into the classification of leukemia types by analyzing the expression of specific surface markers on leukemic cells.30,32 This highly sensitive technique allows for the detection of specific antigens, known as cluster of differentiation markers, which can distinguish between various subtypes of leukemia. For instance, it can differentiate between B-cell and T-cell acute lymphoblastic leukemia by identifying lineage-specific markers like CD19 for B-cells or CD3 for T-cells. Moreover, in cases where distinguishing between acute myeloid leukemia and ALL is essential, flow cytometry offers clarity by identifying myeloid or lymphoid lineage markers, enabling tailored treatment strategies. This precise classification not only aids in diagnosis but also guides therapeutic decision-making, prognostication, and monitoring of minimal residual disease during treatmen.28,32
Imaging test
Imaging tests, including ultrasound, X-rays, and CT scans, play a supportive role in the diagnosis and management of leukemia, particularly when assessing the extent of disease involvement beyond the bone marrow and blood. Although leukemia primarily affects blood and bone marrow, it can spread to other organs such as the spleen, liver, and lymph nodes, leading to complications that imaging can help identify.27 These tests are especially valuable when there is uncertainty in the diagnosis or when physical symptoms suggest the involvement of extramedullary sites.29 For instance, an ultrasound may reveal an enlarged spleen (splenomegaly) or liver (hepatomegaly), which are common in advanced leukemia. Similarly, CT scans can provide detailed images to detect lymph node enlargement or infiltrations in other organs, helping to evaluate the spread or complications such as infections, bleeding, or organ dysfunction. Although imaging is not a primary diagnostic tool for leukemia, it becomes essential when tracking disease progression, staging, or addressing specific clinical concerns during treatment.31
Treatments for leukemia
Leukemia treatments vary widely depending on the type of leukemia, its progression, the patient's overall health, and genetic or molecular features of the disease.33 The main goals of treatment are to eradicate leukemic cells, restore normal blood production, and achieve long-term remission or cure.34 The primary treatment modalities include chemotherapy, targeted therapy, immunotherapy, stem cell transplantation, and radiation therapy. Here is an overview of these treatments.
Chemotherapy
Chemotherapy remains a central treatment for various types of leukemia, particularly acute forms such as acute lymphoblastic leukemia and acute myeloid leukemia.35 It involves the use of cytotoxic drugs that target and kill rapidly dividing leukemic cells. In treating acute leukemias, chemotherapy is administered in several phases.33 The first phase, known as induction therapy, aims to bring about remission by reducing leukemic cells to undetectable levels.34 Following this, consolidation and maintenance therapies are used to eliminate any remaining leukemic cells and prevent relapse. The specific combination and intensity of chemotherapy drugs are tailored based on the leukemia subtype, as well as factors like the patient’s age and genetic risk profile. Although chemotherapy can be highly effective, it often comes with serious side effects, including bone marrow suppression, a higher risk of infections, and potential organ damage.34
Targeted therapy
Targeted therapies have significantly advanced the treatment of various types of leukemia by focusing on specific molecular abnormalities that drive the cancer's growth. These therapies work by interfering with particular proteins or signaling pathways that are essential for the survival and proliferation of leukemic cells, making them more precise and often less toxic than traditional chemotherapy.35 One of the most well-known examples of targeted therapy is the use of Tyrosine Kinase Inhibitors, such as imatinib (Gleevec) and dasatinib, in the treatment of chronic myeloid leukemia.33 These drugs target the BCR-ABL fusion protein, a product of the Philadelphia chromosome translocation, which is responsible for driving the abnormal proliferation of leukemic cells. The introduction of TKIs has transformed CML from a previously fatal disease into a manageable chronic condition for the vast majority of patients.
In acute myeloid leukemia, where FLT3 mutations are common and associated with a poor prognosis, FLT3 inhibitors like midostaurin and gilteritinib have been developed to specifically target the mutated FLT3 gene, significantly improving outcomes for patients harboring these mutations.34 Another area of innovation in AML treatment involves targeting mutations in the IDH1 and IDH2 genes.33 Drugs such as enasidenib and ivosidenib, which inhibit these mutant proteins, have demonstrated effectiveness in treating patients with these specific mutations.35
The advantage of targeted therapies lies in their precision; by honing in on cancer-specific pathways, they tend to spare normal cells, leading to fewer side effects compared to traditional chemotherapy, which indiscriminately kills all rapidly dividing cells. This targeted approach not only improves survival outcomes but also enhances the quality of life for many leukemia patients by minimizing treatment-related toxicity.35
Immunotherapy
Immunotherapy has emerged as a transformative approach in the treatment of leukemia by harnessing the body's own immune system to recognize and eliminate cancer cells. This treatment modality comes in several forms, each designed to enhance the immune response against leukemia.36,37 Monoclonal antibodies are one of the earliest forms of immunotherapy used in leukemia treatment. Drugs like rituximab and ofatumumab are engineered to specifically target proteins on the surface of leukemia cells, such as CD20, which is commonly found in chronic lymphocytic leukemia.34,37 These antibodies bind to the surface proteins, marking the cancer cells for destruction by the immune system. This targeted approach improves the immune system's ability to identify and attack leukemia cells with greater precision.38
Another groundbreaking advancement in immunotherapy is Chimeric Antigen Receptor T-cell therapy.37,38 In this innovative treatment, a patient's own T cells are extracted and genetically modified in the laboratory to express chimeric antigen receptors that recognize and bind to specific markers on leukemia cells.39 Once infused back into the patient, these modified T cells can seek out and destroy cancer cells. CAR T-cell therapy has shown remarkable success, particularly in acute lymphoblastic leukemia, and has been especially effective in treating pediatric patients with relapsed or refractory disease.37,38 The FDA-approved CAR T-cell therapies, such as tisagenlecleucel, have demonstrated impressive remission rates in patients who previously had very limited treatment options.36
Additionally, the exploration of immune checkpoint inhibitors is expanding the landscape of immunotherapy in leukemia. These drugs work by blocking immune checkpoints molecules like PD-1 that cancer cells exploit to evade the immune system.39 By inhibiting these checkpoints, drugs can enhance the immune system's ability to detect and destroy leukemia cells.37 While immune checkpoint inhibitors have revolutionized the treatment of solid tumors, their role in leukemia is still being investigated through clinical trials. This promising research aims to unlock further potential in harnessing the immune system to combat various forms of leukemia.37,38
Stem cell transplantation (Bone marrow transplant)
Stem Cell Transplantation is a potentially curative option for patients with high-risk leukemia or those who have relapsed after initial treatment. This advanced procedure, often referred to as allogeneic stem cell transplantation, involves replacing the patient’s diseased or damaged bone marrow with healthy hematopoietic stem cells from a compatible donor. The goal is to restore the patient’s ability to produce healthy blood cells, which are critical for immune function and overall health. The procedure offers a dual benefit: the healthy stem cells regenerate the patient’s blood and immune systems, while also attacking any residual cancer cells through the graft-versus-leukemia effect. This GVL effect is a form of immunotherapy, where the donor's immune cells recognize and destroy leukemia cells that remain after chemotherapy or radiation therapy.34
Stem cell transplantation is primarily used to treat acute myeloid leukemia, acute lymphoblastic leukemia, and, in certain cases, chronic lymphocytic leukemia and chronic myeloid leukemia. It is often considered for patients with high-risk genetic features, poor response to initial treatments, or those who experience relapse after conventional therapies. The procedure is most beneficial when there is a matched donor, often a sibling or an unrelated donor from a bone marrow registry. In some cases, haploidentical transplants (using half-matched donors, such as parents or children) or cord blood transplants may be considered as alternative donor sources. While stem cell transplantation offers a potential cure, it is associated with considerable risks and challenges.34 One of the most serious complications is graft-versus-host disease, in which the donor's immune cells attack the patient’s healthy tissues. GVHD can range from mild to life-threatening, affecting various organs, including the skin, liver, and gastrointestinal tract.34 Additionally, patients are at high risk for severe infections due to prolonged immunosuppression following the transplant. Other long-term complications can include organ damage, fertility issues, and an increased risk of secondary cancers. Thereore, patients undergoing stem cell transplantation require close monitoring and long-term follow-up care.35
Despite these risks, stem cell transplantation remains one of the most powerful and potentially curative treatments for leukemia, particularly in cases where other therapies have failed. Advances in donor matching, supportive care, and management of complications like GVHD have improved outcomes, making this procedure a critical component in the treatment of high-risk leukemia cases. However, the decision to pursue stem cell transplantation must be carefully weighed against the potential risks, and it is typically reserved for patients with aggressive disease or poor prognosis following standard treatments.34
Radiation therapy
Radiation therapy, which utilizes high-energy radiation to destroy cancer cells or shrink tumors, plays a specific role in the treatment of leukemia.40 Although it is not a primary treatment for most types of leukemia, it is commonly used in certain situations. One of its main applications is in preparing patients for stem cell transplantation, where radiation helps eliminate leukemic cells in the bone marrow, making room for the healthy donor stem cells to engraft. Additionally, radiation therapy is sometimes used to target leukemic cells in specific areas, such as the brain or spinal cord, particularly in patients with acute lymphoblastic leukemia who have central nervous system involvement.40 In these cases, radiation helps control the disease and prevent further spread. While its use in leukemia is limited, radiation therapy can be valuable for managing symptoms and controlling disease progression in certain clinical scenarios.41
CRISPR techniques
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) technology has emerged as a revolutionary tool in genetic engineering, allowing for precise editing of the genome.42 In leukemia, CRISPR has the potential to target and correct specific genetic mutations that drive the disease, offering new avenues for treatment (31). The technology works by utilizing a guide RNA that directs the CRISPR-associated proteins, such as Cas9, to a specific location in the genome.43 Once there, the Cas protein creates a double-strand break in the DNA, which can then be repaired in a way that either disrupts the faulty gene or corrects the mutation.
One of the most promising applications of CRISPR in leukemia is the ability to directly target and disrupt oncogenes that are essential for leukemic cell survival. For example, CRISPR has been used to knockout the BCR-ABL1 fusion gene in Ph+ ALL cells, effectively halting the growth of these leukemic cells in preclinical models. Similarly, CRISPR can be used to restore the function of tumor suppressor genes that are inactivated in leukemia, such as TP53, thereby reinstating normal cell cycle control and apoptosis in leukemic cells.42
CRISPR technology is also being used to explore the genetic basis of drug resistance in leukemia. By systematically knocking out individual genes in leukemic cells, researchers can identify genes that contribute to resistance against targeted therapies, such as TKIs or monoclonal antibodies. This knowledge can then be used to develop combination therapies that prevent or overcome resistance. Additionally, CRISPR screens can identify genes that sensitize leukemic cells to certain drugs, providing insights into potential new therapeutic targets.43
CRISPR is playing a crucial role in the next generation of CAR T-cell therapies for leukemia. One application involves using CRISPR to knock out the endogenous T-cell receptor and other immune checkpoint genes in T-cells before engineering them to express a CAR. This creates universal CAR T-cells that are less likely to cause graft-versus-host disease and are more effective in attacking leukemic cells. CRISPR can also be used to enhance the persistence and functionality of CAR T-cells, potentially improving their efficacy in treating relapsed leukemia.42
Genetic heterogeneity
Leukemia is characterized by significant genetic heterogeneity, both between different patients (inter-patient heterogeneity) and within the leukemic cell population of a single patient (intra-tumoral heterogeneity).41 This heterogeneity arises from the accumulation of genetic mutations, chromosomal aberrations, and epigenetic changes that occur during disease progression. Genetic heterogeneity plays a critical role in disease behavior, influencing the aggressiveness of the leukemia, its response to treatment, and the likelihood of relapse.40
The presence of multiple subclones within a leukemic population, each harboring distinct genetic mutations, contributes to disease progression and resistance to therapy. As leukemia evolves, more aggressive subclones may emerge, driven by selective pressures such as chemotherapy.41 For example, in AML, the acquisition of mutations in genes like FLT3, NPM1, and DNMT3A can lead to more aggressive disease and a higher propensity for relapse. In chronic lymphocytic leukemia, genetic heterogeneity is associated with varying rates of disease progression, with certain mutations (e.g., TP53, NOTCH1) linked to poor prognosis and rapid disease progression.
Genetic heterogeneity also significantly affects the response to treatment.41 The presence of specific mutations can confer resistance to certain therapies, leading to treatment failure. For example, in Ph+ ALL, the T315I mutation in the BCR-ABL1 gene renders the leukemia resistant to first- and second-generation TKIs, necessitating the use of more potent inhibitors like ponatinib. In CLL, the loss of TP53 function is associated with resistance to chemotherapy and poor response to monoclonal antibodies like rituximab.
Moreover, intra-tumoral heterogeneity means that while one subclone may be sensitive to a particular therapy, others may survive and eventually cause relapse. This clonal evolution under therapy is a major challenge in the treatment of leukemia, as it often leads to the emergence of drug-resistant disease. Understanding the genetic landscape of leukemia at diagnosis and during treatment is therefore crucial for selecting the most appropriate therapies and for developing strategies to prevent or overcome resistance.40
The impact of genetic heterogeneity on treatment response underscores the importance of personalized medicine in leukemia care. Genetic profiling, using techniques such as next-generation sequencing, allows for the identification of the specific mutations and chromosomal abnormalities present in a patient’s leukemia. This information can guide the selection of targeted therapies that are most likely to be effective against the patient’s particular disease subtype and can help in predicting the likelihood of resistance.41
Furthermore, genetic profiling can identify patients who may benefit from more aggressive treatments, such as allogeneic stem cell transplantation, or those who might respond to novel agents being tested in clinical trials.40 By tailoring treatment to the genetic makeup of each patient’s leukemia, personalized medicine aims to improve outcomes and reduce the risk of relapse.
Clinical significance
Leukemia, a type of cancer affecting the blood and bone marrow, holds significant clinical relevance due to its impact on the hematopoietic system.44 This disease disrupts the normal production of white blood cells, red blood cells, and platelets, which can lead to symptoms such as anemia, increased infection risk, and impaired clotting ability. Clinically, leukemia is categorized into four main types: acute lymphoblastic leukemia, acute myeloid leukemia, chronic lymphocytic leukemia, and chronic myeloid leukemia, each differing in its pathophysiology, progression, and demographic prevalence.45
Acute leukemias are characterized by rapid progression and an accumulation of immature white blood cells, requiring immediate medical intervention.44,46 They often present with sudden onset of symptoms such as fatigue, bruising, and fever, significantly impacting patient quality of life and necessitating aggressive treatment strategies such as chemotherapy, targeted therapy, or stem cell transplantation.45 On the other hand, chronic leukemias generally progress more slowly and may remain asymptomatic for extended periods, allowing for strategic treatment approaches that balance disease management with patient well-being. 44,46
The clinical significance of leukemia also extends to its genetic and molecular underpinnings.45 Recent advancements have highlighted specific genetic mutations and chromosomal abnormalities associated with different leukemia types, such as the Philadelphia chromosome in CML or mutations in the FLT3 gene in AML. These discoveries have paved the way for targeted therapies that improve survival rates and quality of life, emphasizing the importance of personalized treatment plans.44
Understanding leukemia's impact on patients, including potential complications such as tumor lysis syndrome, organ infiltration, and treatment-related side effects, is crucial for developing comprehensive care plans. Early detection, effective treatment strategies, and continuous research into novel therapies remain central to improving clinical outcomes for leukemia patients. Furthermore, supportive care focusing on symptom management and maintaining quality of life is an integral component of comprehensive leukemia treatment, underscoring its multidimensional clinical importance.44
Leukemia remains one of the most significant challenges in cancer treatment due to its complexity and the diverse forms it takes. The overproduction of abnormal white blood cells that defines leukemia impairs the body’s ability to produce healthy blood cells, leading to complications such as anemia, bleeding disorders, and weakened immunity. This article underscores the crucial advances made in understanding the molecular biology of leukemia, which have led to more effective and targeted treatments.
One key aspect is the identification of genetic mutations that drive the progression of leukemia. For instance, mutations like FLT3 in acute myeloid leukemia or the Philadelphia chromosome in chronic myeloid leukemia have paved the way for targeted therapies. Tyrosine kinase inhibitors such as imatinib for CML and FLT3 inhibitors for AML have revolutionized treatment by targeting specific molecular abnormalities. However, despite these advances, the challenges of drug resistance and relapse persist. Mutations like T315I in BCR-ABL-positive acute lymphoblastic leukemia have made some leukemias resistant to first- and second-generation TKIs, requiring the development of more potent drugs like ponatinib.
Immunotherapy, particularly Chimeric Antigen Receptor T-cell therapy, represents a significant breakthrough in leukemia treatment. By genetically modifying a patient’s T cells to target leukemia cells, CAR T-cell therapy has shown remarkable success, especially in pediatric patients with relapsed or refractory ALL. However, aggressive forms of leukemia, such as T-cell leukemias, continue to present significant challenges, and long-term remission remains difficult to achieve in some patients.
The importance of stem cell transplantation as a potentially curative option for high-risk patients. While this procedure can be life-saving, it comes with considerable risks, such as graft-versus-host disease and a high risk of infections due to prolonged immunosuppression. The continued refinement of this treatment, alongside advancements in supportive care, remains essential for improving patient outcomes.
Furthermore, CRISPR technology has emerged as a promising tool in leukemia research. CRISPR allows for precise editing of the genome and has the potential to correct genetic mutations that contribute to leukemia’s development. This approach has shown promising results in preclinical models, such as knocking out the BCR-ABL1 fusion gene in Ph+ ALL cells, effectively halting the growth of these leukemic cells.
In conclusion, while significant strides have been made in the treatment of leukemia, challenges such as drug resistance, genetic heterogeneity, and relapse must still be addressed. The future of leukemia treatment lies in the continued exploration of personalized therapies, including the development of more advanced targeted treatments, immunotherapies, and CRISPR-based approaches. Ongoing research is essential to overcome these obstacles and improve long-term outcomes for patients affected by this complex disease.
None.
Authors declare that there is no conflict of interest.

©2025 Patel, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.