Journal of
eISSN: 2572-8466


Review Article Volume 7 Issue 4
Correspondence: Raquel Elisa da Silva-López, Researcher of Department of Natural Products, Institute of Pharmaceuticals Technology, FIOCRUZ, Rio de Janeiro, Brazil,
Received: July 19, 2020 | Published: August 24, 2020
Citation: Teixeira EMGF, Oliveira D, Silva-López RE. Plant protease inhibitors as specific strategies against cancer cells. J Appl Biotechnol Bioeng 2020;7(4):169-173. DOI: 10.15406/jabb.2020.07.00230
Cancer is a disease that cause important mortality in the world and investigations in order to understand the mechanisms of tumor development and new treatments are crucial and are carried out in many laboratories worldwide. One of the mechanism of growth and progression of tumors is the secretion of proteases which are responsible for many processes during the cancer progression and metastasis. In this context, molecules that can inhibit these proteases are important strategies for cancer treatment. Plants are important sources of many molecules with a great diversity of functional, structural and pharmacology properties, such as, the protease inhibitors. This review addresses some aspects about molecular mechanisms of cancer proteases and some effects of plant protease inhibitors on cancer cells.
Keywords: cancer, protease, protease inhibitors, plant protease inhibitors
TCA, trichloroacetic acid; EMT, epithelial-mesenchymal transition; ECM, extracellular matrix
Cancer is the second cause of deaths in the world. There are more than 100 diseases that have similar features of uncontrol cell growth. Cancer cells divide rapidly, causing tumors, which can spread to other regions of the body and the different cancer types correspond to the various types of cells in the body. Many studies have shown that proteases, which are enzymes responsible for the cleavage of peptide bonds, are involved in events of growth, development, tumor progression and metastasis. They are essential for the survival of all living organisms, as they mediate signals of initiation, transmission and termination of many cellular events such as growth, development and maintenance of tissues, inflammation, apoptosis, senescence, coagulation, among others.1
Tumoral proteases
Tumors are able to stimulate the expression of proteases in normal neighboring cells, further favoring the growth of tumors.2,3 Various tumor types secrete proteases that participate in various processes related in tumor progression such as cell proliferation, due to the release of growth factors anchored to the extracellular matrix (ECM), immune responses, inflammatory cell recruitment, invasion, angiogenesis, metastasis, apoptosis, epithelial-mesenchymal transition (EMT), response to chemotherapy. Six classes of proteases may be involved in the development and invasion of cancers which are metalo, cysteine, serine, threonine, glutamic and aspartic proteases.1,3,4 Matrix metalloproteases (MMPs) are zinc dependent endoproteases and participate in the synthesis and degradation of a wide variety of ECM components, releasing several biologically active proteins such as cytokines, growth factors and chemokines anchored in membranes.5 There are more than 25 MMPs such as collagenases, gelatinases, stromelysins, matrilysins, membrane-bound MMPs and all associated with different types of tumors. The expression of these proteases in normal tissues is low, however, is greatly increased in most of malignancies, as they have an important proteolytic action in the invasion and metastasis processes.6
Cathepsins are generally cysteine proteases found in lysosomes and perform proteolysis of both intracellular proteins and ECM proteins. They act to promote metastasis to other tissues, blood and lymph vessels. Cathepsins are usually involved with some type of tumor, being tumoral markers such as colorectal, pancreatic, carcinoma of the tongue, lung, breast, gastric, colon, head and neck carcinomas, gliomas, melanomas, as well as cancer endometrial.4,7,8 The activation of zymogens such as pro-MMPs and pro-cathepsins is performed by proteases of the plasminogen activator-urokinase system (uPA). It comprises two serine proteases, urokinase (uPA) and tissue plasminogen activator (t-PA), the cognate uPAR receptor and two endogenous inhibitors: the plasminogen activator inhibitor (PAI-1) and the activator inhibitor of plasminogenio II.9,10 This system also promotes the degradation of the basement membrane and other ECM components, facilitating the expansion of the tumor mass. Cancers of the breast, stomach, colorectal, esophagus, pancreas, gliomas, lung, kidney, prostate, uterus, ovary, liver and bones exhibit a direct correlation between levels of the uPA system with tumor aggressiveness and decreased survival of their hosts. This system is widely studied as a target for anti-cancer therapies,11 Other serine proteases are also crucial in the development of tumors such as Matriptase-2, a transmembrane enzyme, which in addition to degrading the components of ECM, activates the uPA system and is involved in signaling angiogenesis.12,13 A transmembrane serine protease, TMPRSS4, is related to the expressive invasive character of tumors of pancreas, ovaries, thyroid, colorectal, lung, breast, uterus, vesicle, stomach and liver.14 Trypsin is the most well-known serine protease and is involved in many physiological and pathological processes. She is involved in processes of proliferation, invasion and metastasis in colorectal cancer.15,16 Digestion of type II collagen by trypsin promotes invasion of the basement membrane. In addition, the activation of trypsin in tumor cells and the co-expression of MMPs facilitate invasion and metastasis.17 Hepsin or TMPRSS1 is a transmembrane serine protease found mainly in the kidney, pancreas, stomach, prostate and thyroid and its levels are elevated in prostate, breast, ovary, kidney and endometrial cancers. The representative of aspartic proteases is cathepsin D (CATD), which is an important endoproteinase involved in different physiological processes and signaling pathways. It is one of the main proteins secreted in different types of cancers, being present in its microenvironments, where it has been seen to increase its expression and secretion in cancers such as ovarian, breast, endometrial cancer, lung cancer, malignant glioma, melanoma and prostate cancer. Many studies show that the presence of this protease increases the capacity for invasion and the risk of metastasis.19,20
Protease inhibitors in cancer
Protease inhibitors (PIs) are molecules that bind in a specific way at the active site or elsewhere in the enzyme, interfering with catalysis, interrupting or decreasing the cleavage reactions of peptide bonds. The vast majority of natural PIs have one or more polypeptide chains and can be found in plants, microorganisms and animals that control the activity of their target proteases. They can be classified according to the type of proteases that they inhibit in serine, cysteine, aspartic and metalloprotease inhibitors.21 As previously reported, proteases are extremely important in the development, growth of cancers, and can be targeted for drug development. In this way, its specific inhibitors, can be seen as possible anti-cancer agents, acting in the inhibition of these enzymes. MMPs are inhibited by tissue inhibitors of mammalian metalloproteases, TIMPs. They are proteins of 184-194 amino acids with 21kDa. They are: TIMP-1, TIMP-2, TIMP-3 and TIMP-4, each of which inhibits the activity of MMPs with different catalytic efficiencies.1,22 The altered expression of TIMPs shows that they have an important role in tumor environment. Many studies have shown that the high expression of TIMP-1 is associated with a poor prognosis of cancer in the lung, brain, prostate, breast, colon and several other cancers. This expression may be a tissue response to functionally high protease activity in the tumor microenvironment. This inhibitor is being studied as a future biomarker for recognizing or monitoring the response to patients' treatment. Differently, TIMP-3 expression is reduced in many types of cancers, being associated with the advanced stage in colorectal, breast, brain and bladder cancer. TIMP-2 and TIMP-4 can be more or less expressed depending on the type of cancer and the stage of the disease.23
Some MMP-inhibiting drugs have been developed, such as Batimastate and marimastate. Batimastate is a peptideomimetic MMP inhibitor that showed high inhibitory activity against MMPs. But this medicine showed, orally, low bioavailability. Marimastate has advanced to phase 2 and 3 clinical trials for different tumors, such as pancreas, lung, breast, colorectal, brain and prostate. Despite this, the drug caused joint pain, stiffness and inflammation in patients, which forced them not to continue their studies.24 The family of cysteine protease inhibitors, cystatins, appears to have important blocking activity for tumor metastasis and invasion, as they inhibit cathepsins B, H and L which are known for their great importance in the growth, development and metastasis of tumors.25–27 Cystatins A and B, or Stefans A and B, are markers of aggressive tumors that appear in much lower concentrations than in normal individuals.28,29
In normal cells, matriptases (serine proteases) are inhibited by hepatocyte growth factor activator inhibitor-1 (HAI-1) and it has been observed that in prostate cancer, there is a decrease in HAI-1 and increased expression of these proteases. Serpin, inhibitors of serine proteases, are used as markers of colorectal, lung and prostate cancers and inhibit tumor growth and lung cancer angiogenesis.13 As can be seen, tumor cells modulate the activity of proteases involved in extracellular proteolysis, MMPs and uPA/uPAR/plasminogen. In addition, the increase in different tumor proteases, results in the degradation of ECM, the growth of tumors (by releasing growth factors), metastases, angiogenesis, among other events. Therefore, PIs can be considered crucial strategies in anti-cancer therapy, and it is necessary to search for new PIs that inhibit proteases involved with cancers.1
Plant protease inhibitors
Plants are known sources of a huge diversity of molecules, and are also a great source of PIs. Vegetables are susceptible to changes in environmental conditions and, therefore, synthesize molecules for their protection, among them small molecules such as secondary metabolites and larger molecules of primary metabolism, polypeptides, often PIs.30,31 They are important in defense against pests, insects and even pathogens because they are induced in plants through attacks by insects or pathogens. In insects, they interfere in the digestion process because they have the ability to inhibit their digestive proteases. They are low molecular weight polypeptides that are very stable at variations in temperature and pH.32 Plant PIs (PPIs) are found in plants of various systematic groups in particular from species of the Fabaceae or Leguminosae family and accumulate in the seeds, but are also observed in other plant organs. They represent the largest group of plant PIs and their main families include: Kazal, Kunitz, Tomato, Cereal, Soy trypsin inhibitor, Squash, Bowman-Birk, Potato and Serpinas. PPIs have two main functions: mobilization of nutrients such as essential amino acids, peptides and proteins by blocking the degradation of storage proteins in seeds, and protecting the plant from attack by pests by inhibiting their digestive enzymes. These PPIs could have different biological activities and, therefore, are considered as innovative alternatives in the treatment, control and prevention of different types of diseases.33,34 As mentioned before, PIs have many biological functions and they have been investigated as potential strategies for different pathologies, as in different types of cancers. PPIs have been isolated and characterized for several years and have been shown to be good therapeutic agents.35 Studies in animal models have shown that the ingestion of legumes rich in Bowman-Birk family inhibitors (BBI), such as soy, peas, lentils and chickpeas, and the ingestion of the isolated inhibitor prevented and suppressed carcinogenic and inflammatory processes ofgastrointestinal tract.34 The table 1 shows some PPIs and their activity against tumor cells of different types of cancers.
|
|
Origen |
Activity |
Reference |
|
BBI |
Glycine max L. |
Reduced the proliferation of colon adenocarcinoma cell lines |
36, 37, 38 |
|
KTI |
Glycine max L. |
Inhibited uPA urokinase activity of human ovarian cancer cells, decreasing their invasion |
39,40 |
|
BbKI |
Bauhinia bauhinioides |
Inhibited plasma kallikrein, bovine trypsin, chymotrypsin. Decreased pulmonary edema in rabbits |
41,43 |
|
- |
Bauhinia rufa |
Inhibited plasma kallikrein, bovine trypsin and decreased pulmonary edema in rabbits |
42,43 |
|
- |
Cicer arietinum L. |
Decreased the viability of brain cancer and prostate cancer cells |
44 |
|
- |
Coccinia grandis |
Inhibited the growth of colon cancer cell lines |
45 |
|
- |
Elusine coracana |
Reduced cellular proliferation and induced apoptosis of myeloid leukemia cell and not was cytotoxic to human peripheral blood mononuclear cells |
46 |
|
EcTI |
Enterolobium contortilisiliquum |
Inhibited cancer lineages as colloretal, leukemia, breast and prevented proMMP activation |
47 |
|
LC-pi I, II, III, and IV |
Lavatera cashmeriana Camb. |
Demonstrated in vitro anticancer activity on leukemia, lung, and colon cells |
48 |
|
LCTI |
Lens culinaris |
Inhibited cell growth of colon adenocarcinoma |
49 |
|
MsTI |
Medicago scutellata L. |
Citotoxic to breast and cervical carcinoma cells |
50,51 |
|
TBPI |
Phaseolus acutifolius A. and Phaseolus vulgaris L. |
Decreased cell invasion capacity, extracellular matrix degradation and suppressed of MMP-9 |
52 |
|
FBPI |
Vicia faba L. |
Suppressed skin carcinogenesis and stopped pulmonary metastasis of melanoma cells |
53, 54 |
|
BTCI |
Vigna unguiculata |
Affected breast cells |
55,56 |
|
CrataBL |
Crataeva tapia Bark |
Inhibited U87 cell invasion and adhesion |
57 |
|
CC-PI |
Cajanus cajan |
Inhibited cell growth of adenocarcinomic human alveolar |
58 |
Table 1 Plant protease inhibitors with potential application against cancer cells
PPIs (Plant protease inhibitors) extraction
Plant cells are made up of several compounds, including a set of polysaccharides, which makes them difficult to break down. The first step in the preparation of a plant extract rich inproteins, is the disruption of this tissue. It is performed by spraying with grade and pistil using liquid nitrogen. This method reduces the degradation of proteins in the vegetal tissues by minimizing proteolysis and other types of degradation. The finer the powder produced, the more proteins are extracted later (Figure 1A).59,60 Finally, the extraction of proteins is a difficult stage to choose because of the enormous diversity of structural characteristics among plants. There are many protocols for the extraction of proteins for each type of plant and plant tissue and extractions with water and aqueous buffers can be used.32–59 The extraction using phenol and trichloroacetic acid (TCA) showed high yield of protein extraction. In addition, extraction by protein precipitation with TCA and acetone can also be used. Another method used is extraction with buffered phenol (pH 8.0 buffer) followed by precipitation with methanol.
Many methods of isolating and purifying proteins from protein extracts can be used andchromatography is the method of choice (Figure 1B&1C). This technique is use for separating, identifying and purifying mixtures of substances through the interaction between these substances between the mobile phase and the stationary phase. The most employed chromatographies are: affinity, ion exchange and gel filtration. Affinity chromatography is based on the principle of biological affinity, where the protein is isolated according to its function. The specific ligand for the protein to be isolated will be coupled in the stationary phase and they will interact. Those remaining molecules (which are not of interest) are eluted with the stationary phase.45,46 Molecular exclusion chromatography (or filtration gel) is based on a separation in which smaller molecules penetrate the pores of the stationary phase, taking longer to be excluded and larger ones do not penetrate these pores, having a migration speed greater, being excluded first.45,48,52,58 The ion exchange chromatography occurs through the ionic bond that occurs between the charged molecules of the complex sample and the stationary phase, which has an opposite charge.45,47,52,56
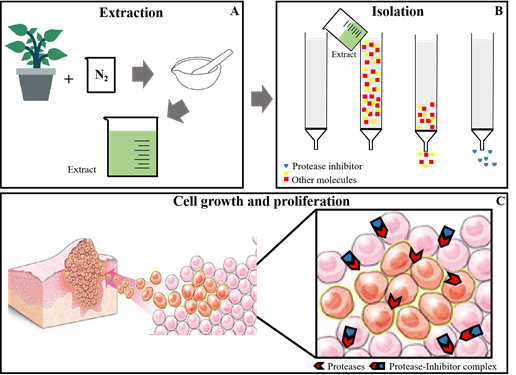
Figure 1 PPIs obtaining: The first step is preparation of extract, plant organ is sprayed with grade and pistil in N2, the proteins in the powder are extracted with different extracting agents (A); isolation and purification is performed with chromatographic techniques (B) and these purified PPIs are evaluated for their biological activity (C).
The investigation of new compounds for cancer treatment is an important challenge in worldwide public health. The proteases are paramount in cancer progression, because they are involved in tumor nutrition, digestion of ECM to release growth factors and facilitate proliferation and metastasis. Consequently, their specific inhibitors can be effective to discontinue tumor cells growth, and could be employed as important agents in the chemotherapy of a great variety of neoplasms. Many plants PIs are polypeptides widely research for a great diversity of cancers, such as breast and colorectal adenocarcinomas. These natural peptides have shown effectiveness, specificity and safety molecules in comparison to the most aggressive and small synthetic molecules recommended for cancer treatments. These PIs bond specifically by multiple points at the active sites of tumor proteases and block the cancer progression. Thus, this review shows the importance of studying these molecules as potential strategies for controlling the growth of cancers.
None.
The author declares that there is no conflict of interest.
None.

©2020 Teixeira, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.