Journal of
eISSN: 2572-8466


Research Article Volume 11 Issue 4
1Department of Biology, Federal University of Technology, Nigeria
2Department of Plant Science and Biotechnology, Adekunle Ajasin University, Nigeria
3Department of Biology, Morgan State University, USA
Correspondence: Akinbode Foluso Ologundudu, Department of Biology, School of Life Sciences, Federal Univeesity of Technology, Akure, Nigeria, Tel +14438577691
Received: August 10, 2024 | Published: August 26, 2024
Citation: Ologundudu AF, Omooye E, Makinde A. Photosynthate, heavy metals contents, biochemical characteristics and anatomical responses in Telfairia occidentalis. and Amaranthus hybridus collected within akure metropolis. J Appl Biotechnol Bioeng. 2024;11(4):130-134. DOI: 10.15406/jabb.2024.11.00369
Heavy metals in soils pose a potential threat to the environment and can cause significant damage to human and animal health. This study aimed to assess heavy metal concentrations, photosynthate levels, biochemical characteristics, and anatomical responses in Telfairia occidentalis and Amaranthus hybridus collected within Akure metropolis, Ondo State, Nigeria. Fresh vegetable samples were randomly collected from four fertilized farmlands (denoted as A, B, C, and D) within the Akure metropolis. Heavy metal concentrations in the vegetable samples were determined using an Atomic Absorption Spectrophotometer (AAS). The pigment content (Chlorophyll a and b) of the vegetables was measured, while the activities of Catalase (CAT), Superoxide Dismutase (SOD), and reduced glutathione (GSH) were estimated. Stomatal architecture and epithelial cell structure were also analyzed.
Results indicated that CAT (7.45 ± 0.36 U/mg protein) and SOD (6.21 ± 0.28 U/mg protein) activities, as well as GSH content (4.63 ± 0.12 µmol/g tissue), were highest in vegetables collected from farm A, showing statistically significant differences compared to other farms (p < 0.05). The concentrations of heavy metals in the leaves of vegetables collected from all farms were below the standard limits recommended by WHO/FAO, with the exception of Lead (Pb), which was significantly higher than the permissible limit in farm B (2.14 ± 0.09 mg/kg; p < 0.05). Chlorophyll content was significantly reduced in both vegetables compared to the control (chlorophyll a: 2.56 ± 0.21 mg/g tissue in T. occidentalis and 2.03 ± 0.19 mg/g tissue in A. hybridus; p < 0.05). This reduction in chlorophyll content correlated with the elevated levels of Pb in farm B (r = -0.87, p < 0.05). The stomatal architecture analysis revealed significant alterations in stomatal density and pore size in both vegetables from contaminated farms.
These findings suggest the need for biomonitoring programs to control heavy metal accumulation in Nigerian farmlands, as elevated levels of Pb can disrupt the physiological and biochemical processes of vegetables, posing potential health risks to consumers.
Keywords: health risk, pigment, biochemical, photosynthate
Contamination of the environment by heavy metals is a global occurrence which adversely affects both human and animal health.1 Heavy metals in soil pose serious threats to the environment and can cause severe damage to human health through various absorption pathways such as direct ingestion, dermal contact, diet through the soil-food chain, inhalation, and oral intake.2 Soil is the primary reservoir of heavy metals in the atmosphere, hydrosphere and biota, and thus plays a fundamental role in the overall metal cycle in nature.3 Industrialization, urbanization and intensification of agricultural activities are the major causes of heavy metal contamination of soils and the environment.
In addition, the indiscriminate and uncontrolled applications of fertilizers and pesticides to agricultural land have also contributed to the continuous accumulation of heavy metals in soils.4 Prolonged application of excessive chemical fertilizers and organic manures on farmlands are also sources of heavy metal of heavy metals in soils.5 Heavy metals, especially cadmium (Cd), are present in some inorganic fertilizers at high concentrations. The presence of cadmium in some fertilizers at high concentrations is of most concern due to the toxicity of this metal and its ability to accumulate in soils and its ultimate bioaccumulation in plants and animals.6 For example, superphosphate fertilizer contains trace metal impurities such as Cd in addition to major elements necessary for plant nutrition and growth. These heavy metals can accumulate in the soil and eventually taken up by plants.7
Vegetables cultivated on contaminated soils absorb heavy metals, leading to their accumulation in the food chain. According to Al Jassir et al,.8 vegetables, especially those of leafy vegetables, cultivated in heavy metals contaminated soils, accumulate higher amounts of metals than those grown in uncontaminated soils. Heavy metal absorption through the food chain by living organisms has been widely reported globally.9 When soil contaminated with heavy metals is used for production and consumption of crops, they are built up in vital organs.10 The accumulation of heavy metals in these vital organs often result in severe health disorder in human body owing to their non-biodegradable nature.11
However, plants respond differently to nutrients and metal concentrations through their changes in pigment concentration, water content, dry weight and growth. The summation of all the plant responses results in distinct light absorption and reflectance characteristics and invariably lead to soil contamination. Numerous studies have shown that metal and nutrient stress in plants give rise to changes in the spectra reflectance of the vegetation12 leading to several physiological and biochemical changes in plants, which can significantly alter the availability of nutrients in the vegetables. Toxic levels of heavy metal enhance production of reactive oxygen species (ROS) including superoxide dismutase, catalase and hydroxyl radicals.13 Heavy metals in the soil reduce the uptake of minerals and micronutrients by plants, interferes with plant water balance, inhibits stomata opening, and decreases plant quality.14 Heavy metals inhibit chlorophyll and carotenoid production in plants but affect the production of chlorophylls more than that of carotenoids.15 Thus, this research was designed to assess the heavy metal contents, photosynthate, biochemical characteristics and anatomical responses in Telfairia occidentalis. and Amaranthus hybridus collected within Akure metropolis, Ondo State, Nigeria.
Study area: The study was carried out in Akure, Ondo State, Nigeria. It lies on latitude 70 15N of the equator and longitude 50 11 42E of the greenwich meridien (Figure 1).
Sample collection
Fresh samples of Telfairia occidentalis. and Amaranthus hybridus were randomly collected from four cultivated fertilized farmlands (A, B. C and D) in Akure metropolis, Ondo State, Nigeria.
Experimental design
The control experiment was set up in a screen house at the Federal University of Technology, Akure. Representative samples were harvested in the morning and transported directly to the laboratory for analysis. The samples were collected in triplicates.
Determination of photosynthetic pigment contents
The pigment content (chlorophyll A and B) of the two vegetables was determined in the laboratory using the spectrophotometric method (Merzlyak et al., 1996).
Sample preparation
All the collected vegetable samples were washed with distilled water to remove airborne particles, air dried to remove the moisture and water droplets, and pulverized to fine powder using a laboratory grinder. The pulverized vegetable samples were sieved using Whatman Filter Paper (2mm). One gram each of the vegetable sample was weighed using a electronic balance (Model: WT5002) into a 50 ml beaker, 5 ml of analytical grade acid HN03 and HCl added. The mixture was allowed to stay for 5 minutes, and heated at a temperature of about 80-90 0C for one hour thirty minutes using electric hot plate (HP 220, UTEC products Inc., Albany N.Y., USA) until a clear solution was obtained. After cooling, the solution was made up to a final volume (50 ml) with distilled water in a volumetric flask.
Determination of heavy metals
Metal content in the samples was determined using an Atomic Absorption Spectrophotometer (AAS) (model: Agilent Technology (Spectra 55B), Australia). Analysis of each sample was replicated and results reported in mg/kg.
Biochemical characteristics
The activity of superoxide dismutase (SOD) in the homogenates was determined according to the method described by Misra et al,.16 Catalase activity (CAT) was carried out according to the method described by Hadwan17 while the method described by Beutler et al., (1963) was adopted in the estimation of the level of reduced glutathione (GSH) in the testis and brain supernatants.
Anatomical characteristics
Stomata Architecture and epithelial cell structure was carried using the method described by Yeung (1998).
Statistical analysis
Data collected will be subjected to analysis of variance using Duncan New Multiple Range Test at 0.05 probability level. All statistical analyses were carried out using SPSS 21.0.
There were significant differences in the antioxidant activities of the two sampled vegetables collected from the four cultivated fertilized farmlands (A, B, C and D) in Akure metropolis. Significant variations in catalase activity (CAT) in the two vegetables (Amaranthus hybridus and Telfairia occidentalis.) were observed (Figure 1). Our results showed that the catalase activity was higher and in the two vegetables collected from the four farms. However, significant increase in CAT was observed in vegetables collected from farm A in comparison with the control. Glutathione (GSH) content was highest in both vegetables collected from farm A, followed by the vegetables collected from farm C when compared with the control (Figure 2). Superoxide dismutase (SOD) increased in the two vegetables, but a significant increase was observed in vegetables collected from farm A when compared to the control (Figure 3).
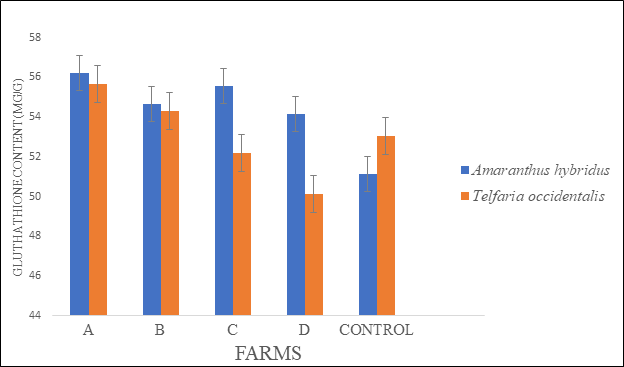
Figure 2 Glutathione (GSH) contents in the leaves of Amaranthus hybridus and Telfairia occidentalis. collected at major farms in Akure metropolis.
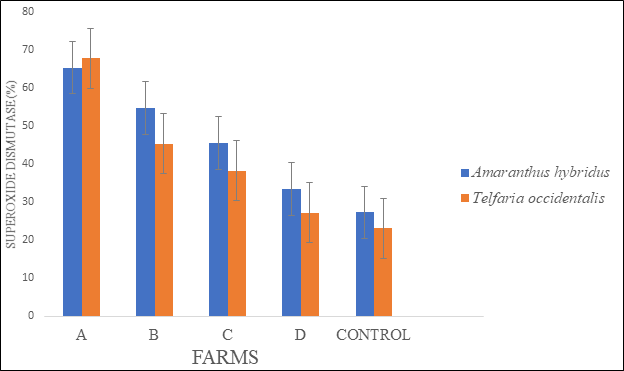
Figure 3 Superoxide dismutase (SOD) activities in the leaves of Amaranthus hybridus and Telfairia occidentalis. collected at major farms in Akure metropolis.
The result of the stomata architecture and epithelial cell structure of the vegetables are presented in Plates 1 and 2. The Foliar anatomy of Amaranthus hybridus collected from Farms A and B showed open stomata (Plate 1). Reduction in stomata was observed in T. occidentalis collected from Farm B (Plate 2).
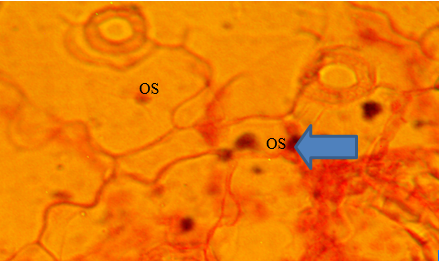
Plate 1 Foliar anatomy of Amaranthus hybridus collected from Farms A and B in Akure metropolis OS=Open Stomata.
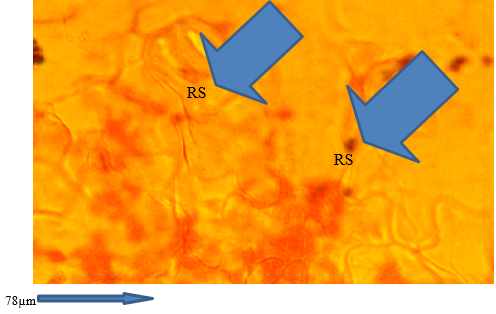
Plate 2 Foliar anatomy of Telfairia occidentalis. with reduced stomata collected from Farm B in Akure metropolis. RS= Reduced stomata.
Differences in the concentrations of heavy metals (Cu, Zn, Mn, Pb and Cd) were observed in A. hybridus collected from the four farms (Table 1). The concentration of Cu was highest (0.193 mg/kg) in A. hybridus collected from farm A while significant increases in concentrations of Zn (3.45 mg/kg) and Mn (2.78 mg/kg) were observed in samples collected from farm D. Highest concentration of Cd (0.10 mg/kg) was shown in leaves of A. hybridus collected from farm A which differed significantly from those collected from farms B, C and D. Lowest level of Pb (0.39 mg/kg) was detected in A. hybridus collected from farm B which did not differ significantly from those collected from other farms (A, C and D). Table 2 presents the concentration of heavy metals in the leaves of T. occidentalis from selected farms A, B, C and D. T. occidentalis collected from farm D showed highest concentration of Cu (0.64 mg/kg) while significantly highest concentrations of Zn (2.90 mg/kg) and Mn (6.06 mg/kg) were observed in T. occidentalis collected from farm B. Similarly, T. occidentalis collected from farm B showed the highest concentration of Pd (0.43 mg/kg) which did not differ significantly from those samples from other farms. Cd (0.013 mg/kg) concentration was highest in T. occidentalis collected from farm C. The concentration of these heavy metals, except Pb, were all below the standard limits recommended by WHO/FAO and National Environmental Standard and Regulation Enforcement Agency (NESREA). Pb concentrations in both vegetables collected from all the farms were all above the safe limit (0.3 mg/kg) set by WHO and FAO.
|
Farms |
Cu |
Zn |
Mn |
Pb |
Cd |
|
A |
0.193±0.02a |
1.58±0.06b |
1.31± 0.02a |
0.40±0.07a |
0.10± 0.01c |
|
B |
0.35± 0.04b |
0.75±0.05a |
1.62±0.01b |
0.39±0.03a |
0.06± 0.00b |
|
C |
0.36± 0.01b |
2.70±0.66c |
2.05±0.07c |
0.40±0.01a |
0.003±0.06a |
|
D |
0.40±0.02a |
3.45±0.70c |
2.78±0.08c |
0.40±0.05a |
0.07± 0.01a |
|
WHO/FAO Safe Limit |
73 |
100 |
200 |
0.3 |
0.1 |
Table 1 Concentration of heavy metals (mg/kg) in leaves of Amaranthus hybridus L. from selected farms in Akure
|
Farms |
Cu |
Zn |
Mn |
Pb |
Cd |
|
A |
0.28±0.03a |
1.58±0.03b |
3.60±0.02b |
0.40±0.04a |
0.003±0.001a |
|
B |
0.29±0.02a |
2.90±0.01c |
6.06±0.06c |
0.43±0.03a |
0.001±0.00a |
|
C |
0.62±0.03b |
0.84±0.01a |
2.23±0.07a |
0.40±0.01a |
0.013±0.01a |
|
D |
0.64±0.03b |
1.57±0.03b |
3.70±0.02b |
0.40±0.02a |
0.011±0.01a |
|
WHO/FAO Safe Limit |
73 |
100 |
200 |
0.3 |
0.1 |
Table 2 Concentration of heavy metals (mg/kg) in leaves of Telfairia occidentalis from selected farms in Akure
Means followed by similar alphabet are not significantly different (P>0.05) using DMR test
The chlorophyll contents reduced significantly in the two vegetables collected in comparison with the control (Figure 4).
Results of our study revealed varied concentrations of heavy metals of Copper (Cu), Zinc (Zn), Manganese (Mn), Lead (Pb) and Cadmium (Cd) in the leaves of the two selected vegetables from the four cultivated fertilized farmlands (A, B. C and D) in Akure metropolis, Nigeria. In order to establish possible contamination and accumulation of heavy metals, vegetables were considered to be contaminated when values of heavy metals were above the standard limits recommended by WHO/FAO and National Environmental Standard and Regulation Enforcement Agency (NESREA). In this study, the range of concentrations of heavy metals in the leaves of vegetables collected from the farms were below the standard limits recommended by WHO/FAO and National Environmental Standard and Regulation Enforcement Agency (NESREA). The results showed that Cu ranged from 0.193-0.40 mg/kg in A. hybridus to 0.28-0.64 mg/kg in T. occidentalis. Zn ranged from 0.75-3.45 mg/kg in A. hybridus to 0.84-2.90 mg/kg in T. occidentalis. Mn ranged from 1.31-2.78 mg/kg in A. hybridus to 2.23-6.06 mg/kg in T. occidentalis. Pb ranged from 0.39-0.40-0.40 mg/kg in A. hybridus to 0.40-0.43 mg/kg in T. occidentalis while Cd ranged from 0.003-0.10 mg/kg in A. hybridus to 0.001-0.013 mg/kg in T. occidentalis. However, the concentration of Pb in both vegetable species sampled from selected vegetable farms were above the threshold recommended by WHO/FAO. This is in accordance with the earlier work of Osundiya et al,.18 who reported highest value of Pb (24.07mg/kg) in leafy vegetables while working on bioaccumulation of heavy metals in frequently consumed leafy vegetables in Nigeria. According to Igwegbe et al,.19 the higher levels of heavy metal contamination found in some vegetables could be closely to over usage of fertilizers in farms. Lead poison is very severe, and it normally gains entrance to the body biochemical system through the air, water and food. According to Mohammed et al,.20 Pb accumulation in some plants can surpass several hundred times the threshold of maximum level acceptable for human intake.
Our results also revealed increases in the activities of anti-oxidative enzymes in the leaves of the two vegetables. Results showed increases in the catalase activity (CAT), Glutathione (GSH) content and Superoxide dismutase (SOD) in the leaves both vegetables in comparison to the control. According to Anahita et al,.21 there is a correlation between heavy metal resilient plants and antioxidant activity. They opined that antioxidant potential was higher in heavy metal resilient than in non-resilient plants. A significant increase in activity of both the antioxidant enzymes catalase reveals that production of these enzymes might be playing an important role to scavenge the Reactive Oxygen Species (ROS) produced in response to bioaccumulation of heavy metals in the cell. These enzymes are known to play a defensive role against oxidative stress.22 The increase in activity of antioxidant enzymes relates to a similar study conducted by Singh et al,.23 in which a significant increase in the activity of peroxidase and catalase in Beat vulgaris irrigated with different concentrations of sewage waste water was reported.
The reduction in the chlorophyll a and b in the two vegetables could be as a result of the accumulation of Pb. According to Oropeza-Garcia et al,.24 Pb is a metal of very low mobility, often most of its portion is reserved in soil at the root level. However, small proportions of Pb may inhibit respiration and photosynthesis due to the disturbance of electron transfer chain reaction.25 The considerable decrease of chlorophyll in this study, supports the assertion that the chloroplast is the primary site of attack by contaminants.26
The outcome of this present study revealed that the heavy metal concentration in the leaves of the samples vegetables were lower than the maximum permissible limit according to the international to Food and Agriculture Organization (FAO) and World Health Organization (WHO). However, the concentration of Pb in both vegetable species sampled from the selected vegetable farms were found to be above the threshold recommended by WHO/FAO. Additionally, the increased catalase content in both vegetable sample collected from Farm A confers structural stability to cellular machinery by acting as osmolyte capable of detoxifying reactive oxygen species (ROS) towards ameliorating heavy metals accumulation. The increased GSH content in Amaranthus hybridus and Telfairia occidentalis. in Farm A possibly indicate that vegetables are capable of maintaining high degree of cell membrane homeostasis under heavy metals accumulation. The accumulation of chlorophyll a and b in both vegetables samples showed inconsistent pattern, photosynthesis is indirectly affected by heavy metals accumulation in leaves which influences the functioning of the stomata and therefore affects transpiration rates and overall growth of the plants. Reduced photosynthesis under heavy metals accumulation is not only attributed to stomata closure leading to a reduction in intercellular CO2 concentration, but also to non-stomata factors. Therefore, continuous monitoring of heavy metals in vegetables consumed by the populace is warranted. In addition, there is an urgent need for the establishment of biomonitoring programs. Future studies will focus on phytoremediation to mitigate the accumulation of heavy metals in soils.
None.
The authors declare no conflicts of interest.
None.

©2024 Ologundudu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.