Journal of
eISSN: 2572-8466


Research Article Volume 7 Issue 5
Department of Biotechnology Engineering, International Islamic University Malaysia, Malaysia
Correspondence: Faridah Yusof, Department of Biotechnology Engineering, Faculty of Engineering, International Islamic University Malaysia, P.O. Box 10, 50728 Kuala Lumpur, Malaysia, Tel 603-64214597, Fax 603-64214442
Received: September 13, 2020 | Published: October 13, 2020
Citation: Al Safi NAAA, Yusof F, Azmi AS. Involvements of food grade dialdehydic pectin as cross-linker and soy protein as additive in the production of MNP- CLEA-lipase from Hevea brasiliensis. J Appl Biotechnol Bioeng 2020;7(5):215-223. DOI: 10.15406/jabb.2020.07.00236
Skim latex from Hevea brasiliensis (rubber tree) contains many useful enzymes that can be utilized to produce value-added products. Lipase recovered from skim latex serum was immobilized via cross-linked enzyme aggregates (CLEA) technology, while supported by magnetic nanoparticles termed ‘MNP-CLEA-lipase’. One of the main advantages of this method is, the biocatalyst can be easily recovered by a magnetic field for recycling. In this research, dialdehydic pectin was used as the cross-linking agent, replacing glutaraldehyde, and soy protein was used as an additive, replacing bovine serum albumin (BSA). The operating conditions for the preparation of the most active MNP-CLEA-lipase were optimized statistically. Dialdehydic pectin and soy protein showed optimum operating conditions at 180 mg/mL and 0.6% (w/v) respectively and with (NH4)2SO4 at 80% saturation, giving a Residual Activity (RA) of 154%. The optimum temperature for MNP-CLEA-lipase was at 40°C (127% RA) while the optimum pH was at 8 (127% RA). It retained 20% RA after 100 days of storage. The reusability test showed that it maintained 7% RA after the 10th cycle. This research showed two advantages; first, that value-added products can be recovered from agricultural waste, and second, by alternating to low-cost food-grade cross-linkers and additives, enhanced biocatalyst can be produced which can be safely used in the beverage and food industries.
Keywords: MNP-CLEA-lipase, Hevea brasiliensis, cross-linked enzyme aggregates (CLEA), macromolecular cross-linker, glutaraldehyde, pectin, soy protein
CLEA, cross-linked enzyme aggregates; MNP, magnetic nanoparticles; RA, residual activity; BSA, bovine serum albumin; FCCCD, face centred central composite design; RSM, response surface methodology; FESEM, field emission scanning electron microscopy
Natural rubber (cis-1,4-polyisoprene) of Hevea brasiliensis is highly used in the latex-based industry to produce such products like tires, latex gloves, latex threads, and rubber catheters.1 As a basic raw material in rubber processing, fresh field latex undergoes many procedures during its conversion to dry rubber or high concentrated latex, whereby, the processing usually involves three methods: centrifugation, creaming, and evaporation.2–4 Some of the processes have been carried out by chemical treatment that poses a lot of negative impacts on the environment.2 High concentrated latex is prepared by treating the natural rubber with ammonia and then centrifuged to separate the aqueous part from the concentrated rubber. This aqueous part is called skim latex which is one of the most polluting wastes in the rubber industry.5 Before being discharged into the main water system, skim latex is usually pre-treated in the effluent treatment oxidation pond.1 Converting skim latex into valuable substances would become an alternative to minimize the discharging of effluent into the main waterways, thus making latex processing more environment friendly.
Lipid acyl hydrolase, a subtype of lipase, is one of the potential proteins that can be recovered from the latex serum.5 This enzyme has been immobilized by Cross-Linked Enzyme Aggregates (CLEA) technology which is a carrier-free immobilized enzyme technique for biocatalysis.6,7 Traditionally, CLEA preparation involves protein precipitation using precipitants such as ammonium sulphate [(NH4)2SO4], followed by the cross-linking process by cross-linkers such as glutaraldehyde. CLEA combines purification and immobilization into a single operation to provide highly stable and recyclable catalysts with remarkable catalytic efficiency.8 However, CLEA only works well when the enzyme has enough lysine (amine residues) which links the enzyme and the cross-linker. Insufficient cross-linking might lead to enzyme leaching into the reaction media and poor stability.9 CLEA processing usually involves separation by centrifugation or filtration which would end up as clumps due to low compression resistance. This may have inhibited the internal mass transport of substrate and hence, reduce the catalytic activity.10 Magnetic cross-linked enzyme aggregates (magnetic CLEAs) is a new improvement in CLEA technology which has been proven to enhance the catalytic activity of CLEA-immobilized enzymes by increasing its cross-linking ability which leads to improved stability and catalytic activity.10–12 For the preparation of magnetic CLEAs, amino-functionalized magnetic nanoparticles (MNPs) are added into the enzyme solution for immobilization. There are two great advantages to this method. First, the functionalized MNPs add more amine residues to generate more cross-linking of the enzyme aggregates which increases the mechanical stability of CLEAs and produces non-leachable CLEAs. Second, the magnetic properties of CLEAs help in the separation of this biocatalyst from the reaction mixture using a magnetic field, whereby this process solves the clump formation problems caused by centrifugation or filtration and makes recycling the immobilized enzyme much easier.10,11
Cross-linking agents play important role in locking the enzyme aggregates to form the solid biocatalyst. Glutaraldehyde is the common type of cross-linker used for CLEA technology due to its low price and availability in commercial quantities.8 However, glutaraldehyde is not compatible with certain enzymes such as nitrilases which leads to low activity retention. Also, glutaraldehyde’s small-sized molecule and high reactivity allows it to leach into the enzyme’s active site which would lead to low activity retention.8,10 Nadar et al.,9 and Gupta et al.,13 discovered that macromolecular cross-linker such as dialdehydic pectin can replace glutaraldehyde in CLEA-immobilization while increasing the immobilized enzyme’s activity. Pectin is not a natural cross-linker and for it to become a cross-linker, aldehydic carbonyl functional groups are introduced to the molecules by periodate oxidation. Using pectin, a food-based molecule in the preparation of biocatalyst is encouraging because it would not harm the environment as well as can be safely used in food and beverage industries.
Another compound that may be added in the preparation of CLEA, to stabilize and enhance the enzyme’s activity is additives. There are three main categories of additives commonly used in enzyme immobilization, such as proteic co-feeders, organic mediums, and surfactants. Each of these additives affects the final immobilized enzyme differently, but positively enhancing their activities. Protein co-feeder is commonly added to the CLEA mix when the enzymes are known to be low in concentration. Low enzyme concentrations may result in inadequate cross-linking and this may lead to CLEA with low stability and easily leachable, thus a second protein added, such as bovine serum albumin (BSA), could improve the CLEA structure by providing additional lysine residues to react with the cross-linker.14 Additives can also prevent the leaching of cross-linkers into the enzyme’s active site.14,15 As for organic medium, for example, n-heptane has been used by Guauque Torres et al.,16 which resulted in higher CLEA activity. This is mainly due to the increase in CLEAs interfacial activation leading to better interaction between the enzyme and the substrate as well as with the cross-linker.16 And finally, anionic surfactant may be added to CLEAs preparation, which aims in facilitating the precipitation of enzyme into a stable conformation.17 Gupta et al.,17 observed an efficient cross-linking with an increase in CLEA activity in the presence of sodium dodecyl sulfate (SDS) by two folds. Moreover, surfactants preferentially interact with some of the binding sites of an enzyme or form more powerful hydrophobic bonds than existing ones and thus changing the enzyme structure, thereby affecting the activity.18 However, in this study, we are focusing on enhancing CLEA by adding proteic co-feeder by using food grade products. Since common proteic additive such as BSA is expensive and can increase the final cost of the biocatalyst,19 Karimpil et al.,20 discovered a cheaper food-grade additive, soy protein, which can replace BSA as an additive in CLEA-immobilization. Soy protein is rich in lysine residues which can facilitate cross-linking of enzymes.
In this research, dialdehydic pectin as the cross-linker and soy protein as a proteic additive are used in the production of the food-grade biocatalyst. The preparation conditions of this biocatalyst are optimized statistically using Face Centred Central Composite Design (FCCCD) under Response Surface Methodology (RSM) with the aid Design Expert® software (Version 11). This biocatalyst could be a discovery in producing eco-friendlier and more cost-effective MNP-CLEA immobilized enzymes with enhanced stability, targeting the food and beverage industries.
Preparation of skim latex serum
Glacial acetic acid was added to skim latex to reduce the pH from 10 to 5, upon which the rubber particles coagulated. Then, the acidified sample was centrifuged at 10 000 rpm at 4°C for 30 min to separate the supernatant (skim latex serum) from the coagulated rubber (skim rubber). Solid (NH4)2SO4 was added to the pooled supernatant, up to 4 M concentration, and left to precipitate overnight under slow stirring at 4°C. Then, the samples were centrifuged at 5000 rpm for 15 min and the collected precipitate was dissolved in minimal phosphate buffer saline (PBS) at pH 7.5.
Protein assay
Protein assay was conducted according to Bio-Rad Bradford,21 by using the micro-assay method. The linear range of the assay for standard BSA is 0.1 to 1.0 mg/mL. 800 µL of each standard and sample solution was pipetted into a clean, dry test tube. Protein solutions were assayed in triplicates. 200 µL of diluted dye reagent was added to each tube and vortexed. The samples were incubated at room temperature for 5 min. The absorbance of the samples was measured at 595 nm using Thermo Scientific Multiskan Go™ Spectrophotometer. The protein content was calculated based on the equation from the standard curve obtained.5
Lipase enzyme activity assay
Substrate stock solution was prepared by dissolving 28 mg of p-nitrophenyl palmitate (pNPP) in Triton 100-X (100 mL, 1% v/v) and SDS (1.7mL, 1% w/v) while stirring and heating. To start the assay reaction, 1 mL of pNPP stock solution was incubated with Tris-HCl (1 mL, 0.1 M, pH 8.2) and 1 mL of the enzyme in a water bath for 30 min at 37°C. NaOH (1 mL, 1 M) was added to stop the reaction and vortexed. The absorbance of the samples was measured at 410 nm. One unit of lipase enzyme activity is defined as the amount of enzyme required to release 1.0 µmol of p-nitrophenol per minute under the assay conditions.22,23Lipase activity was calculated using Eq. (1).
(1)
Specific activity is calculated according to Eq. (2):
(2)
Preparation of magnetic nanoparticles
Ferric chloride (FeCl3.6H2O, 1.351 g) and ferrous sulphate (FeSO4.7H2O, 0.6852 g) were added to 25 mL deionized water. Ammonium hydroxide (NH4OH) was added to the solution until a precipitate was obtained at room temperature. To remove the residual ions, the precipitate was centrifuged and washed several times with deionized water until a pH 7 was obtained. The precipitate was dried at 100oC for 3 h to obtain dried magnetite nanoparticles.12 The surface of the particles is coated with 3-aminopropyl triethoxysilane (APTES) by silanization reaction to obtain amino-functionalized MNPs (fMNPs). The silanization reaction involved dissolving 1 mL of APTES, 2 g of MNPs, and 0.25 mL of deionized water in 25 mL of methanol. The mixture was sonicated for 30 min. Glycerol (15 mL) was added to the mixture, and the solution was incubated at 80°C for 6 h at 200 rpm. The precipitate obtained was washed with water and methanol for three times in each case and dried, yielding a fine powder.10,14,17 For working MNP suspension, 20 mg of fine fMNPs was suspended in PBS.
Preparation of dialdehydic pectin
The periodate oxidation process was conducted to introduce aldehydic carbonyl functional groups to the pectin molecules. Pectin was dissolved in a volume ratio of 80:20, water: ethanol. For oxidation, sodium meta periodate (NaIO4) (3 mL, 0.5 M) was added and the pH of the solution was adjusted to 3.5 using dilute HCl and sodium bicarbonate (NaHCO3) solution. Oxidation was carried out under constant stirring for 3 h in the dark at 60°C. Ethylene glycol (3 mL) was added to stop the oxidation. Then, the oxidized pectin was precipitated out by adding excess isopropanol and separated by vacuum filtration.13,24–26The precipitate was dried at 40°C overnight.24 Before being used as a cross-linker, dried dialdehydic pectin was dissolved in sodium acetate buffer (0.1 M, pH 6.0) to make 0.42 mol/L aldehyde content which is equivalent to glutaraldehyde.13,24–26
Preparation of MNP-CLEA-lipase
To prepare MNP-CLEA-lipase, 20 mg amino-functionalized MNP was first mixed with 1 mL of free lipase enzyme solution. Then, varying amounts of (NH4)2SO4, dialdehydic pectin, and soy protein were added to the mixture of enzyme solution to make a final volume of 4 mL solution.27 The solution was agitated at 200 rpm for 17 h at room temperature. The immobilized enzymes were washed three times using distilled water and separated from the reaction mixture using a magnet. The RA of MNP-CLEA-lipase was obtained by conducting the lipase enzyme activity assay.10 The RA of CLEA-lipase was determined using Eq. (3).
(3)
Optimization of condition parameters for preparing MNP-CLEA-lipase
The optimum condition parameters for preparing MNP-CLEA-lipase were experimentally determined using FCCCD under RSM with the aid Design Expert® software (Version 11). The software helps to determine the interactions between each parameter used in the experiment. The parameters involved were concentrations of dialdehydic pectin as cross-linker, (NH4)2SO4, and soy protein as additive. A total of 20 experiment runs were prescribed including six replications at the center point. Each run was conducted in triplicates and an average was reported. Statistical analysis was done using ANOVA to evaluate the degree of accuracy of the derived model equation.
Validation experiments
Three validation experiments were carried out to check on the authenticity of the model experiment. The reliability of the model experiment was given by the percentage error of the predicted and the actual value of RA of MNP-CLEA-lipase, calculated using Eq. (4).
(4)
Determination of optimum temperature and pH
To determine the optimum temperature for MNP-CLEA-lipase, the lipase enzyme activity assay was conducted at different incubation temperatures (25 to 60°C). To measure the optimum pH, lipase enzyme activity assay was conducted as usual but at optimum temperature and at various pH (5 to 10).27,28 The optimum temperature and pH were determined according to the highest RA obtained.
Thermal and pH stabilities
For the thermal stability test, MNP-CLEA-lipase was incubated in a substrate-free buffer at optimum pH for 30 min at temperatures between 25°C to 60°C. After incubation, the samples were brought back to room temperature and the substrate was added. Then, the samples were incubated again at the temperature for 30 min. As for the pH stability test, MNP-CLEA-lipase was incubated in a substrate-free buffer at various pH ranging from 5 to 10 at the optimum temperature for 30 min. After 30 min, the substrate was added, and the samples were incubated at optimum temperature for another 30 min.6
Storage stability
Storage stability was determined by storing MNP-CLEA-lipase in sodium acetate (NaCH3CO2) buffer (0.1 M, pH 5) without substrate at 4°C. Lipase enzyme activity assay was carried out every five days for 100 days.13 The RA of MNP-CLEA-lipase for Day 1 was set as 100%.
Reusability test
The enzyme reusability test for MNP-CLEA-lipase was done by recycling MNP-CLEA-lipase for 10 consecutive cycles. The assayed MNP-CLEA-lipase (first cycle) was washed and stored in 3 mL of water at 4°C for 24 h, whereby the assay was repeated nine more times. The RA was calculated by taking the enzyme activity of the first cycle as 100%.6
Morphology analysis by FESEM
Field Emission Scanning Electron Microscopy (FESEM) analyses were conducted to analyze the structure of MNP-CLEA-lipase. The appearance of the sample could either be spherical (Type 1) or less-structured (Type 2).29 MNP-CLEA-lipase and non-functionalized MNP samples were sent to Crest Nanosolutions (M) Sdn. Bhd., Puchong, Selangor, for FESEM analysis. The FESEM was operated at 10 kV using FEI Quanta FEG. The samples were dried and placed on an aluminium stab, then coated with gold particles before being scanned under vacuum.
Results
Optimization of preparation condition for MNP-CLEA-lipase
Results (not shown) obtained from the One-At-a-Time (OFAT) experimental method were used to design the FCCCD experimental runs. OFAT results showed that the best preparation conditions were obtained by using three parameters, namely, the concentrations of dialdehydic pectin, soy protein, and (NH4)2SO4 at 180 mg/mL, 0.6% (w/v) and 80% saturation, respectively. The optimum preparation conditions and the interaction between these three parameters were studied using FCCCD under RSM with the aid of Design Expert® (Version 11) software. In the FCCCD method, the concentrations for dialdehydicpectin were varied at 170, 180 and 190 mg/mL, for soy protein was 0.5, 0.6 and 0.7% (w/v) and for (NH4)2SO4 was 70, 80 and 90% saturation. RA (%) of lipase enzyme activities were recorded as the responses.
The details of the software suggested 20 experimental runs (inclusive of six replications at the center point) are shown in Table 1. The highest RA was obtained at 180 mg/mL of dialdehydic pectin, 0.6% (w/v) of soy protein, and 80% saturation of (NH4)2SO4 with a RA of 154%. Results showed that concentrations of dialdehydic pectin, soy protein, and (NH4)2SO4 affected the lipase enzyme’s RA and were optimum at the center points. The analysis of variance (ANOVA) data in Table 2 showed that the model F-value of 50.48 implies that the model is significant. There is only a 0.01% chance that an F-value this large could occur due to noise. A P-value less than 0.05 indicates that the model terms are significant while values greater than 0.1 indicate that the model terms are not significant.27,30The model terms are presented in coded factors A, B, A2, B2, and C2. The regression model equation for this experimental design is as Eq. (5) which signifies the relationship between the response (RA) and the variables (concentrations of pectin, (NH4)2SO4, and soy protein). The regression equation obtained for the model is as follows:
(5)
|
Run |
Factor 1 |
Factor 2 |
Factor 3 |
Response |
|
A: Concentration of pectin |
B: Concentration of (NH4)2SO4 |
C: Concentration of soy protein |
Residual Activity |
|
|
(mg/ml) |
(% Saturation) |
(% w/v) |
(%) |
|
|
1 |
180 (0) |
80 (0) |
0.6 (0) |
137 |
|
2 |
180 (0) |
70 (-1) |
0.6 (0) |
88 |
|
3 |
190 (+1) |
80 (0) |
0.6 (0) |
75 |
|
4 |
180 (0) |
80 (0) |
0.6 (0) |
156 |
|
5 |
190 (+1) |
70 (-1) |
0.5 (-1) |
65 |
|
6 |
180 (0) |
80 (0) |
0.6 (0) |
142 |
|
7 |
180 (0) |
80 (0) |
0.6 (0) |
154 |
|
8 |
180 (0) |
80 (0) |
0.6 (0) |
145 |
|
9 |
190 (+1) |
70 (-1) |
0.7 (+1) |
49 |
|
10 |
190 (+1) |
90 (+1) |
0.5 (-1) |
46 |
|
11 |
170 (-1) |
70 (-1) |
0.7 (+1) |
17 |
|
12 |
180 (0) |
80 (0) |
0.6 (0) |
142 |
|
13 |
170 (-1) |
70 (-1) |
0.5 (-1) |
33 |
|
14 |
180 (0) |
80 (0) |
0.5 (-1) |
153 |
|
15 |
180 (0) |
90 (+1) |
0.6 (0) |
68 |
|
16 |
170 (-1) |
90 (+1) |
0.7 (+1) |
9 |
|
17 |
180 (0) |
80 (0) |
0.7 (+1) |
141 |
|
18 |
170 (-1) |
80 (0) |
0.6 (0) |
34 |
|
19 |
170 (-1) |
90 (+1) |
0.5 (-1) |
10 |
|
20 |
190 (+1) |
90 (+1) |
0.7 (+1) |
37 |
Table 1 Design of experiment of FCCCD under RSM by design expert® (Version 11)
|
Source |
Sum of Squares |
df |
Mean Square |
F-value |
p-value |
|
|
Model |
56372.21 |
9 |
6263.58 |
50.48 |
< 0.0001 |
significant |
|
A-Concentration of pectin |
2856.1 |
1 |
2856.1 |
23.02 |
0.0007 |
|
|
B-Concentration of ammonium sulphate |
672.4 |
1 |
672.4 |
5.42 |
0.0422 |
|
|
C-Concentration of soy protein |
291.6 |
1 |
291.6 |
2.35 |
0.1563 |
|
|
AB |
7.28E-12 |
1 |
7.28E-12 |
5.86E-14 |
1 |
|
|
AC |
8 |
1 |
8 |
0.0645 |
0.8047 |
|
|
BC |
60.5 |
1 |
60.5 |
0.4876 |
0.5009 |
|
|
A² |
15412.55 |
1 |
15412.55 |
124.22 |
< 0.0001 |
|
|
B² |
7255.11 |
1 |
7255.11 |
58.47 |
< 0.0001 |
|
|
C² |
855.36 |
1 |
855.36 |
6.89 |
0.0254 |
|
|
Residual |
1240.74 |
10 |
124.07 |
|||
|
Lack of Fit |
962.74 |
5 |
192.55 |
3.46 |
0.0995 |
not significant |
|
Pure Error |
278 |
5 |
55.6 |
|||
|
Cor Total |
57612.95 |
19 |
|
|
|
|
Table 2 ANOVA for experimental results of FCCCD by design expert® (Version 11)
Optimum temperature and pH
The optimum temperature and pH of MNP-CLEA-lipase was determined by incubating the enzyme at different temperatures and pH conditions. The optimum temperature obtained for MNP-CLEA-lipase is 40°C with 127% RA as shown in Figure 1. MNP-CLEA-lipase exhibited high RA even at high temperature, showing a RA above 90% at 60°C. As for the pH, the highest RA of MNP-CLEA-lipase was achieved at pH 8 with RA of 127% as shown in Figure 2. It is observed that MNP-CLEA-lipase maintains a high RA even at extreme pH conditions but is optimum at pH 8.
Thermal and pH stability
The thermal stability of MNP-CLEA-lipase was conducted by incubating the enzyme without substrate at different temperatures at the optimum pH before running the standard lipase enzyme activity assay. On the other hand, the pH stability test was carried out by incubating the enzyme without substrate at different pH at the optimum temperature, before running the standard lipase enzyme activity assay. According to Figure 3, MNP-CLEA-lipase maintained its stability even at high temperature, whereby at 60°C the biocatalyst was shown to still retaining 98% RA. Figure 4 shows that MNP-CLEA-lipase has high stability towards extremes pH conditions, retaining a RA up to 99% at pH 5 and 107% at pH 10.
Storage stability
Storage stability was carried out to study MNP-CLEA-lipase’s activity up to 100 days of storage. A batch of MNP-CLEA-lipase samples was stored in sodium acetate buffer (0.1 M, pH 5) without substrate at 4°C. The stored enzymes were assayed every five days to obtain their RA. The RA of MNP-CLEA-lipase for Day 1 was taken as 100%. From Figure 5, the RA of MNP-CLEA-lipase was observed to decrease gradually as it retained a RA of above 50% of its initial activity until day 40, and it continued to decrease to 20% after reaching the 100th day.31–35
Reusability
The reusability test is an important factor for the immobilized enzymes to be used in industrial applications to ensure its cost-effectiveness.10,26,35 The reusability test of MNP-CLEA-lipase was carried out for 10 cycles and the first cycle was set at 100% RA. The results in Figure 6 showed that the RA of MNP-CLEA-lipase decreased continuously in each cycle and only retained 7% of RA on the 10th cycle. However, it is very laudable to have the MNP-CLEA-lipase achieving a RA of 44% at its 4th cycle of usage.
Scanning electron microscopy by FESEM and EDX
Figures 7 showed the observed FESEM images whereby Figure 7(a) illustrates the images of functionalized MNPs before it was added to CLEA-immobilization. The MNPs appeared to be spherical in shape and loosely aggregated. The diameter of the MNPs was estimated at about 20-60 nm. Figure 7(b) shows the image of CLEA-lipase with glutaraldehyde, without the addition of MNPs. The structure of CLEA-lipase was less structured with the formation of clumps. On the other hand, MNP-CLEA-lipase with pectin and soy protein (Figure 7(c))appeared to be less structured but with lesser clumping. The addition of MNPs seems to give an improved modification to the structure of the immobilized lipase due to more sufficient cross-linking. Lastly, Figure 7(d) shows the structure of MNP-CLEA-lipase with glutaraldehyde. It appeared to also be less structured with the formation of clumps on the surface. In EDX, the x-rays are produced by inelastic scattering of primary beam electrons with bound inner shell electrons during their penetration into the matter. The x-rays enable the determination of the elemental compositions of a sample.38 The EDX results (not shown) showed that MNP-CLEA-lipase containing the highest number of oxygen and CLEA-lipase containing the highest number of carbon. MNP-CLEA-lipase was shown to have iron which indicates that the immobilized enzyme was successfully bonded with MNPs. The presence of some foreign elements such as boron indicates some slight contamination in the samples.
According to the ANOVA results, the concentrations of dialdehydic pectin and (NH4)2SO4 are significant model terms as their p-value is less than 0.05. on the other hand, the concentration of soy protein is not a significant model term with as p-value of greater than 0.1. This inferred that the concentrations of soy protein did not have much influence on the RA compared to concentrations of dialdehydic pectin and (NH4)2SO4. This might be due to the smaller range used for soy protein which is 0.5, 0.6 and 0.7% (w/v). The lack of fit value of 3.46 implies there is a 9.95% chance that a lack of fit F-value this large could occur due to noise.27,30 The model is shown to be significant with a Coefficient of Determination (R2) value of 0.9785. The predicted R2 of 0.9188 is in reasonable agreement with the adjusted R2 of 0.9591 since the difference is less than 0.2. Adequate precision that measures the signal to noise ratio is 20.108 which indicates an adequate signal.22,30,31 The regression equation [Eq. (5)] is represented by a three dimensional surface plot (Figure 8) which indicates the interaction between concentrations of dialdehydic pectin and (NH4)2SO4. The concentration of soy protein was fixed at 0.6% (w/v). A dome or maximum shape of the surface plot indicates that the interactions between the variables are significant.22,27,31 The results for the validation experiment is shown in Table 3. The model’s predicted RA value appeared to be slightly lower than the actual RA obtained for the three runs which might be influenced by the experimental conditions. This means that the calculated results were in the zone within the border of the technological space that FCCCD considered.22,30,31 The average percentage error for the actual and predicted RA of MNP-CLEA-lipase is low of 5.01%, which indicates that the model experiment is deemed to be accurate and reliable to predict the activity of MNP-CLEA-lipase.
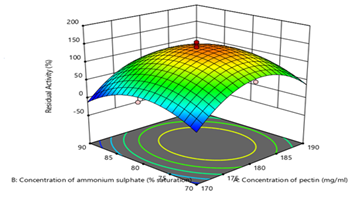
Figure 8 A Three-Dimensional Surface Plot for the Interaction of Concentration of Pectin and Concentration of (NH4)2SO4 by RSM using Design Expert® (Version 11) Software.
|
Run |
A |
B |
C |
Actual Residual Activity |
Predicted Residual Activity |
Error (%) |
|
(mg/ml) |
(%SAT) |
(%W/V) |
(%) |
(%) |
||
|
1 |
184.848 |
85.609 |
0.6 |
66.2 |
62.627 |
5.86 |
|
2 |
182.379 |
72.579 |
0.6 |
122.207 |
116.93 |
4.51 |
|
3 |
184.793 |
77.952 |
0.6 |
135.825 |
129.772 |
4.66 |
|
|
|
|
|
|
Average error |
5.01 |
Table 3 Percentage error for model experiment validation
Optimum temperature experiments showed that MNP-CLEA-lipase can achieve a maximal activity at 40°C (127% RA), and still showed a high RA of above 90% at 60°C (Figure 1). Talekar et al.,10 & Nadar et al.,26 obtained the highest RA of MNP-CLEA-α-amylase and MNP-CLEA-glucoamylase, respectively, at 60°C with residual activities above 90%. In a research carried out by Safi and Yusof32, MNP-CLEA-lipase of skim latex with glutaraldehyde, achieved an optimum temperature of 35°C with 85.89% RA. Thus, MNP-CLEA-lipase with pectin showed more significantly higher RA at optimum temperature. This shows that MNP-CLEA-lipase can work at high temperatures, inferring the significant presence of covalent bonds between the lipase and the dialdehydic pectin during immobilization which decreases the conformational flexibility of lipase and protects it from distortion or damage at high temperature.10,26
MNP-CLEA-lipase showed that it achieved 127% RA at its optimum pH at 8. The results in Figure 2 showed that MNP immobilized lipase has higher activity when dialdehydic pectin and soy protein is added, and the activity is increased even higher than the control (free enzyme). Talekar et al.,10 and Nadar et al.,26 reported an optimum pH of 6 for their work on MNP-CLEA-α-amylase and MNP-CLEA-glucoamylase, respectively. Safi & Yusof32 reported an optimum pH 8 for MNP-CLEA-lipase from skim latex of Hevea brasiliensis, with glutaraldehyde but no additive, however with lower RA (69.92 %). The difference in optimum pH of MNP-CLEA-lipase might be caused by the alteration of acidic and basic amino acid side chain ionization in the microenvironment around the enzyme’s active site. This is also caused by the new interactions formed between basic residues of lipase and pectin during cross-linking.10,26 The higher RA of MNP-CLEA-lipase with pectin compared to with glutaraldehyde might be influenced by pectin’s bigger molecule size which prevents mass transfer limitations and leaching into the enzyme’s active site.9,13
As for the thermal stability, results in Figure 3 show that MNP-CLEA-lipase retained 98% RA at 60oC which is higher than MNP-CLEA-lipase with glutaraldehyde, reported by Safi & Yusof32 (70% RA at 60°C). Talekar et al.10 observed that MNP-CLEA-α-amylase maintained RA of above 85% at 60 to 70°C. The enhanced stability of MNP-CLEA-lipase at high- temperature conditions might be due to enough numbers of covalent cross-linking between lipase enzyme and MNPs as these covalent bonds provide an effective conformational stabilization.10 Figure 4 shows that MNP-CLEA-lipase has high stability at slightly extreme pHs, retaining up to 99% at pH 5 and 107% at pH 10. This indicates that MNP-CLEA-lipase is very stable in acidic and alkaline pH conditions. This is actually more stable to extreme pH’s than MNP-CLEA-lipase (with glutaraldehyde) reported by Safi and Yusof, whereby it only retained less than 60% RA at pH 10 and less than 70% at pH 5.32 The resistance to pH changes of MNP-CLEA-lipase might be caused by the efficient covalent cross-linking of enzyme aggregates with MNPs which increased the flexibility of MNP-CLEA-lipase upon extreme ionization.27,33Having the MNP-CLEA-lipase retaining 50% and 20% after 40 and 100 days, respectively, showed that MNP-CLEA-lipase is significantly stable after a long period of storage (Figure 5). The decrease in RA might be caused by the denaturation of lipase after some period. However, the RA decreased gradually which might be because of minimal distortion effects caused by the buffer solution on the active sites of MNP-CLEA-lipase.9 These results are in good agreement with other work such as Talekar who reported that their MNP-CLEA-α-amylase retained 80% of initial activity after 18 days of storage10 and Kim et al.,34 who reported that their MNP-CLEA-lipase retained 82% RA after 30 days of storage.34 The results implied that magnetic CLEAs are mechanically stable after a long period of storage due to effective cross-linking which prevents leakage of the enzyme into the aqueous buffer and dissociation from aggregates.10,34
Recyclability is another important characteristic of a good biocatalyst. The results in Figure 6 showed the activity of MNP-CLEA-lipase declined to 7% after the 10th usages and this may be caused by loss of enzymes during washing and inactivation of enzymes.10,26,35 Other works showed that recyclability of MNP immobilized enzymes, such as Safi and Yusof’s work32 on MNP-CLEA-lipase with glutaraldehyde, which retained up to 56.46% of RA on the 6th cycle, Talekar work which achieved 25% of RA on the 6th cycle for MNP-CLEA-α-amylase and Talekar et al.,36 work which obtained high retention of 85% RA for MNP-CLEA-glucoamylase after the 10th cycle. The difference in retention of RA for each immobilized enzyme might be influenced by the stability of the enzyme or the cross-linking efficiency of the enzymes.
Field emission scanning electron microscopy (FESEM) is a technique to obtaining structural information on materials at nanometer-scale resolution.37 Energy-dispersive X-ray spectroscopy (EDX) determines the elemental composition of a specimen. FESEM and EDX are important aids in the determination of the relationship among catalyst particles (cluster) sizes, dispersion onto support, morphology, and the influence of promoters.38 FESEM provides imaging of features with high resolution and high contrast in the nanometer scale. Figures 7 show the FESEM images for (a) functionalized MNPs, (b) CLEA-lipase, (c) MNP-CLEA-lipase cross-linked with pectin and added soy protein, and (d) MNP-CLEA-lipase cross-linked with glutaraldehyde. The results showed that MNP-CLEA-lipase with pectin and soy protein appeared to be less structured but with lesser clumping. The addition of MNPs seems to give an improved modification to the structure of the immobilized lipase due to more sufficient cross-linking. The formation of clumps on the surface of the CLEAs should be avoided to ensure better mass transfer. From the FESEM images obtained, it can be said that dialdehydic pectin and soy protein additive works as a better cross-linker and additive for MNP-CLEA-lipase as the result showed improved structure which translates to lesser mass transfer resistance.
According to Schoevaart et al.,29 CLEAs are categorized as Type 1 (spherical shape) and Type 2 (less-structured shape). Non-supported CLEAs can also form large clusters which may cause mass transfer limitations.29 Less structured (Type 2) particles indicate that the structure of the CLEAs was less compact compared to Type 1 structures which are spherical shaped. This is vital for ease of accessibility of larger substrates, such as p-nitrophenyl palmitate with 16 carbon, to the enzymes active site32. The morphology and particle size of CLEAs are also influenced by other parameters such as the concentration of enzymes, type of precipitants, cross-linkers, and additives.39 According to Sheldon39 the particle sizes of CLEAs also affect filterability, especially for non-supported CLEAs. The RA of CLEAs are also influenced by the particle size and the rate of addition of the cross-linker and cross-linking time.39
Nowadays, more attempts should be made to turn any waste into value-added products which will lead to a zero-discharge scenario in many industries. In this case, utilizing skim latex waste for the discovery of biocatalyst can help to reduce the waste management problems faced by the rubber manufacturing industry and will eventually bring benefits towards the industry and the environment. Lipase is chosen as it is a very versatile enzyme that can be used in many industrial applications. The study on MNP-CLEA immobilized enzymes has increased in recent years and is still expanding as it is a very efficient enzyme immobilization method. However, to be able to be utilized in food-based industries and for environmental purposes, green, low-cost and non-toxic materials should be sought for the immobilization technique. Fortunately, low-cost food-grade cross-linkers and additives are proven to be better alternatives that can counter the problems faced by using traditional cross-linker such as glutaraldehyde and costly additives such as BSA while enhancing the enzyme’s biocatalytic activity. Since they are food grade alternatives, the newly produced biocatalyst can be safely used in the food and beverage industries.
The authors would like to thank the Department of Biotechnology Engineering, Faculty of Engineering, International Islamic University of Malaysia for providing the supports to carry out the research.
The authors declare there is no conflict of interest.
None.

©2020 Al, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.