Journal of
eISSN: 2572-8466


Review Article Volume 11 Issue 5
Department of Bioengineering, University of California, USA
Correspondence: Bill Tawil, Department of Bioengineering, UCLA School of Engineering, 420 Westwood Plaza, Room 5121, Engineering V. P.O. Box: 951600, Los Angeles, CA 90095-1600, USA, Fax +(310) 794-5956
Received: August 21, 2024 | Published: September 5, 2024
Citation: Bee-Ling L, Tawil B. Hydroxyapatite bone cement: an in-depth analysis of the process, recent developments, and future considerations. J Appl Biotechnol Bioeng . 2024;11(5):136-144. DOI: 10.15406/jabb.2024.11.00370
Bone cements are used for numerous orthopedic surgeries and other medical procedures. These cements help hold implants in place and provide structural integrity and fill void spaces to enhance the success of implant operations. Hydroxyapatite (HA) is one of the most used types of bone cement with global sales in the hundreds of millions of dollars and a strong expected growth over the next decade as new clinical uses for bone cement are pursued and the products themselves are further developed to mitigate drawbacks to its use.
Keywords: hydroxyapatite bone cement, manufacturing process, composites, market forecast, injectable bone cement, doping
BCIS, bone cement implantation syndrome; HA, hydroxyapatite; CAGR, compound annual growth rate; PMMA, polymethylmethacrylate; PCS, poly(citrate-silicon) hybrid polymer; BSG, borosilicate glass
Bone cement is a commonly used biomaterial for a wide variety of procedures, namely in orthopedic surgery.1 It acts as a space-filler that holds implants against bone.1 Hydroxyapatite (HA) bone cement is constructed from a calcium phosphate preparation.2 It is created by mixing a powder base and a liquid.3 It can be injected directly to an operative site which allows for orthopedic and minimally invasive surgeries.4 It then can be manipulated during operation and will set in vivo to implants composed of microporous HA.3 HA bone cements had global sales of $786.1 million dollars in 2023.5 Sales in North America are estimated to exceed $469 million alone based on a compound annual growth rate (CAGR) of >3% by the year 2033.5 This paper will focus on hydroxyapatite bone cements.
Bone cement is manufactured by combining a solid powder and a liquid monomer.6 Mixing of the solid and liquid causes a polymerization effect to take place by way of free radical reactions and, based on the concentrations of the initiator and the activator, regulated kinetics.6 Bone cements, in general are processed into the human body, this means that rheological properties must be satisfied before the cement sets.6 Bone cements will set in about 15-20 minutes, which gives the surgeon that much time to mold and shape the cement as needed for the current procedure or operation.7 HA bone cement cosmetically performs well, it allows for ease of use, and its pliability allows for surgeons to be precise when molding and applying it during operations.7 HA, chemically represented as Ca10(PO4)6(OH)2, is a valuable material used in bone substitution.8 HA is chemically and crystallographically similar to carbonated apatite that is found in human bones and teeth.8 It exhibits excellent biocompatibility and has demonstrated uses as a bone substitute since 1985 when the first HA bone cement was prepared.8 Once mixed with water the cement forms HA as the only final product, HA is considered the best substitute for hard tissues.8 It is used in a wide variety of fields including dentistry, orthopedics, and plastic surgery.8
Manufacturing process and preparation for use
Manufacturing bone cement begins with creating a polymethylmethacrylate (PMMA) powder.9 The powder is manufactured via a chemical wet precipitation method.9 It is synthesized, decanted, depurated, dried, ground, sifted, pressed, and sintered.9,10 Bone cements are composed of calcium phosphate powders that when combined with water, create a paste that can be easily shaped during operations and procedures.7 Multiple types of calcium phosphate have been used as hard tissue substitutes including hydroxyapatite, tetra calcium phosphate, tricalcium phosphate, dicalcium phosphate anhydrous, amorphous calcium phosphate, and biphasic calcium phosphate(Figure 1).8
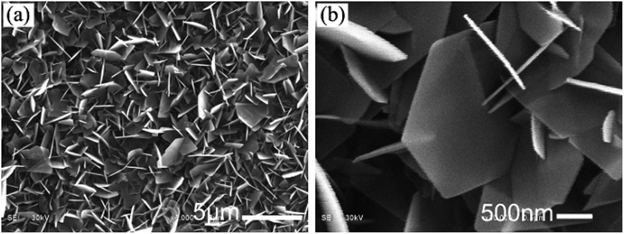
Figure 1 SEM images of HA46
The image shows an SEM image of HA crystals on PA matrix in phosphate solution a) low-magnification b) high-magnification.
HA bone cement begins as polymethylmethacrylate (PMMA) bone cement that is then impregnated with HA.11 The addition of HA to bone cement promotes the growth of bone tissue in pores that are left behind after the HA resorbs into the body.11
Composite bone cement strategies have been researched to improve the properties of bone cement.6 Additives are included to create the composite bone cements for various reasons that can improve them, but medical personnel must be aware of all positive and negative effects.12 Barium sulfate and zirconium oxide for instance are mixed in to increase the detectability of the bone cement through radiological means.12 Sometimes the benefits of certain additives also hamper the bone cement in certain ways, the barium sulfate and zirconium oxide also decrease the mechanical strength of the cement which can cause issues with the loosening of prostheses.12 This then requires the addition of other additives to increase strength, manufacturers can do this by including steel fibers, glass fibers, carbon fibers, and titanium fibers.12 Rubber toughened cement has shown a 167% increase in fracture toughness than non-reinforced bone cement(Table 1).12
|
Additive |
Benefit |
Hindrance |
|
Barium sulfate |
Radio-opacifying |
Mechanical weakness, poor osteoblast formation, lowered biocompatibility |
|
Zirconium oxide |
Radio-opacifying |
Mechanical weakness |
|
Iodine |
Radio-opacifying |
Mechanical weakness |
|
Bismuth salicylate |
Radio-opacifying |
|
|
Gadolinium |
Radio-opacifying |
|
|
Manganese |
Radio-opacifying |
|
|
Steel fiber |
Mechanical strength |
|
|
Glass fiber |
Mechanical strength |
|
|
Carbon fiber |
Mechanical strength |
|
|
Titanium fiber |
Mechanical strength |
|
|
Rubber |
Mechanical strength |
|
|
Stainless steel coil |
Mechanical strength |
|
|
Amphilic bonder |
Interface Integrity |
|
|
Bioactive ceramic powder |
Osteoconduction |
|
|
1-dodecylmercaptan |
Thermal Reduction |
|
|
Ammonium nitrate |
Thermal Reduction |
|
|
Zeolites |
Thermal Reduction |
|
|
Magnesium oxide |
Osteoblast adhesion, radio-opacifying, thermal reduction, minimizes tissue necrosis |
|
|
Erythromycin |
||
|
Gentamycin |
Antibiotic |
Poor diffusion |
|
Tobramycin |
Antibiotic |
|
|
Sodium oxacillin |
Antibiotic |
|
|
Sodium cefazolin |
Antibiotic |
|
|
Vancomycin |
Antibiotic |
|
|
Vitamin E |
Increased cyctocompatibility, cellular proliferation, thermal reduction, lowered bone necrosis |
|
|
PMMA |
Reduces bone cement shrinkage, mechanical strength, fracture toughness, bone growth inducing |
|
|
Multi-walled nanotubes |
Flexural strength, flexural modulus, fracture toughness |
Lowered biocompatibility |
|
Nano-sized titanium fiber |
Flexural strength, flexural modulus, fracture toughness |
Lowered biocompatibility |
|
Silver ions |
Antimicrobial |
|
|
Nanosilver |
Antimicrobial |
|
Table 1 Bone cement additives2
Complications and hazards of bone cement
There are some hazards when dealing with bone cement, the mixing process is exothermic and will generate heat.13 Along with the heat, it creates pungent fumes in the operating room that if inhaled over time can lead to nervous system side-effects similar to being inebriated and if inhaled by pregnant women can cause overexposure to the fetus.14 This requires operating rooms to be well ventilated with laminar air flow systems to remove the fumes.14
Stem-cement debonding is a condition that can increase the stress load of a prosthetic by two to three times.15 Longevity and ultimately the survival of joint replacements is negatively affected, to a detrimental level, by stem-cement debonding.15 Debonding accelerates the failure of a prosthetic by four times and promotes the formation of debris pathways at the interface.16 Debonding of bone cement and the increased stress related to it, causes cracks to form from radial and axial stress.16 The bone cement polymer then easily breaks under the combined shear and compressive stresses.16 This imparts damage to the polymer chain and eventually to the cement-stem structure, causing the interface between the implant and the body to loosen.16
Bone cement implantation syndrome (BCIS) is a condition identified in some patients after undergoing cemented hip hemiarthroplasty.17 BCIS presents as a sporadic and potentially lethal complication that can occur intra- and perioperatively mostly in cases of hip and knee arthroplasties.18 There is currently no standard definition for the syndrome, as it is poorly understood, but it has presented in clinical practices with symptoms like hypoxia, systemic hypotension, pulmonary hypertension, cardiac arrhythmias, loss of consciousness, and cardiac arrest.17,18 Theories do exist as to what causes BCIS, one of the more recent ones is embolus-mediated models.17 Echocardiograms have shown emboli in the right atrium and ventricle as well as the pulmonary artery.17 These emboli have contained fat, marrow, air, bone particles, cement particles, and aggregates of platelets and fibrin.17
Market, companies, and products
In 2023, the global bone cement market was $786.1 million.5 Over the next decade, it is estimated that the industry and market will grow at a CAGR rate of more than 3%.5 North America had a market share of $349 million and the EU had a market of $221.8 million in 2023.19–21
The market is expected to grow over the next decade with the increased popularity of orthopedic treatments with North America leading the worldwide bone cement market.22 The EU will have a moderate expansion as well as the Asia-Pacific market, due in large part to higher estimated demands in nations like China and India, is expected to expand significantly through 2032.22 The Asia-Pacific rise of public awareness and availability of orthopedic treatments is the main driver for this increase.22 Single doses of bone cement in operations can cost between $40 to $185.23 Doses that contain antibiotics to help prevent infections can cost between $117 to $663(Figure 2).23
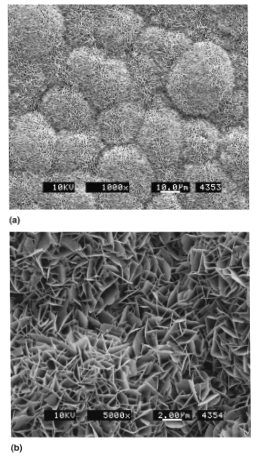
Figure 2 SEM micrographs of carbonated apatite deposits47
The image shoes an SEM image of apatite deposits at a) low and b) magnification.
Stryker has two market-leading hydroxyapatite bone cements that are used for cranial applications, DirectInject and HydroSet (Figure 3).24
DirectInject is the first and only on-demand HA cement.24 It is a self-setting calcium phosphate cement that repairs neurosurgical burr holes, craniotomy cuts, and cranial defects that are not tied to the stability of the bone structure.24 DirectInject does not require any manual mixing and can be applied directly to the site for application of surface areas of 4 cm2 (Figure 4).24 DirectInject won the gold at the US-Ireland Research Innovation Awards in 2017.25 DirectInject received FDA approval in September of 2015.25
HydroSet is a self-setting calcium phosphate bone substitute.24 It is also used in the same instances as DirectInject and can be used in the augmentation and restoration of the contours of the craniofacial bones.24 HydroSet will harden when exposed to water, blood, and cerebrospinal fluid.24 The hardened HA will be replaced by natural bone growth through resorption.24 The hardening process is non-exothermic and removes any issues of heat-related injuries during the setting process.24 It can be applied via spatula, hand, or injected with a syringe to tailor itself to the surgeon’s needs.24 HydroSet received FDA approval in October of 2016(Figure 5).26
Cerament G, manufactured by Bonesupport, is an injectable bone void filler, that contains an antibiotic to protect against gentamicin-sensitive organisms.27 Cerament G has properties that permit surgeons to better manage bone defects in a single-stage procedure (Figure 6).27 Single-stage application also allows limited healthcare resources, namely time, to be used to meet other needs and increases the cost effectiveness of clinics.27 Within a year Cerament G will fully restructure into the host’s bone material, this fast-paced bone remodeling has the benefit of reducing the risks of fracture and infection.27 Cerament G was approved for clinical use by the FDA in May of 2022.28
DePuy Synthes manufactured Cranios Reinforced Fast Set Putty is a calcium phosphate-based biocompatible bone cement that has reinforcement fibers that increase toughness and will set at body temperature (Figure 7).29 The reinforced cement resists fracturing during setting and the sodium hyaluronate additive makes it easier to handle and enhances mixing.29 Cranios Reinforced Fast Set Putty received FDA approval in April of 2006(Figure 8).30
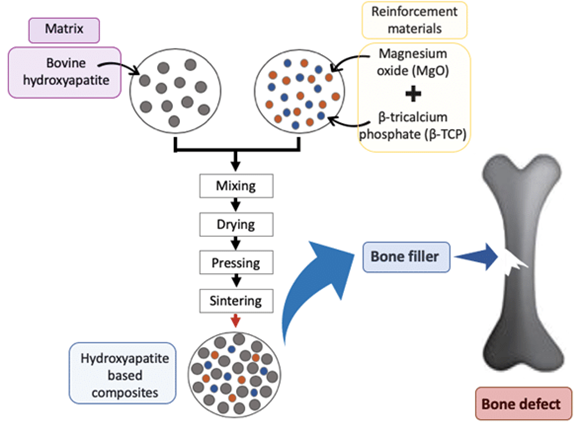
Figure 7 HA bone manufacture and application10
The image shows a graphic illustrating a simplified manufacturing flow.
OtoMimix is manufactured by Olympus.31 Like the Fast Set Putty, it must be manually mixed before application.31 It is used in middle ear implants.28 OtoMimix must be mixed by the operating surgeon before use; it will then allow a surgeon 2-4 minutes to work with it once properly mixed (Figure 9, 10).31 OtoMimix was approved by the FDA for clinical use in November of 2004 (Table 2).32
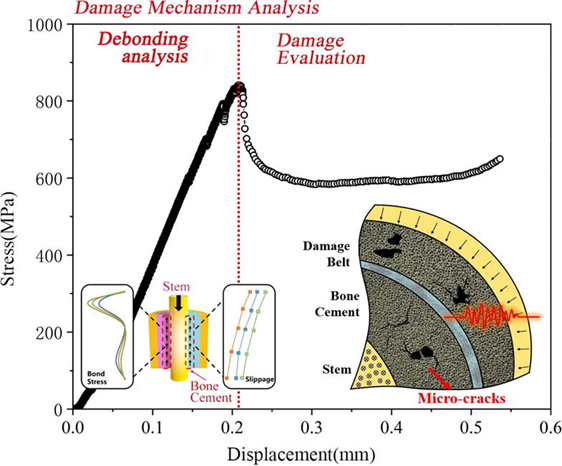
Figure 9 Damage mechanism analysis of debonding of bone cement15
The image shows a Stress vs Displacement graph of damage and debonding of bone cement.
|
Doping ion |
Biologic activity |
|
|
Cation |
Na+ |
Enhanced osteoconductivity and improved cell proliferation |
|
K+ |
Enhanced thermal stability |
|
|
Mg2+ |
Improved crystal growth, crystallization, thermal stability, and dissolution rate |
|
|
Sr2+ |
Enhanced bone regeneration and formation, and inhibited bone resorption |
|
|
Mn2+ |
Enhanced cell adhesion |
|
|
Zn2+ |
Enhanced bone formation and regeneration, increased antimicrobial activity |
|
|
Anion |
CO32- |
High specific surface area, reduced crystallite size, enhanced osteoconductive properties, increased solubility |
|
SiO44- |
Enhanced bioactivity, improved dissolution rate |
|
|
F- |
Improved stability, reduced solubility, and increased remineralization |
|
|
Cl- |
Enhanced osteoconductive properties, and increased solubility |
|
Table 2 Doping material introduced into HA structure and improved properties38
In summary, the market for degenerative disc disease exhibits various segments based on disease indication, including degenerative disc disease, traumas & fractures, complicated deformities, and others. The largest segment is projected to be degenerative disc disease, primarily driven by the increasing number of disc replacement procedures and the growing demand for artificial discs. Promising trends impacting the market include advancements in diagnostic techniques, treatment modalities, emerging technologies, and the adoption of inorganic growth strategies by key market players (Figure 11). These trends contribute to the comprehensive understanding and assessment of the degenerative disc disease treatment market.33–35
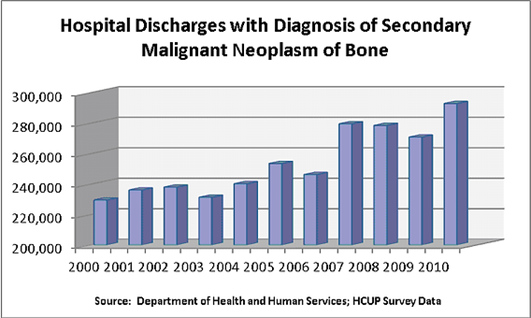
Figure 11 Hospital discharges with diagnosis of secondary malignant neoplasm of bone38
This image shows patient data from the Department of Health and Human Services.
Clinical trials
Clinical trials for bone cement products are still ongoing.33,36,37 There are currently three active trials, two of which are recruiting, studies: Smith and Nephew’s FDA-approved Porous Total Knee System, Insignia Hip Stem Outcomes, and Hyaluronic Acid +Hydroxyapatite vs. Hydroxyapatite in Bone Regeneration.33,36,37
The Smith and Nephew study is looking into the safety and effectiveness of an FDA approved system to replace worn and diseased knee joints (Figure 12).33 The study is hoping to prove that patients who receive this treatment have reduced pain and greater mobility from an implant that is long-lasting.33 This study began in December of 2021 and is estimated to be completed by December of 2034, it has around 500 participants.33
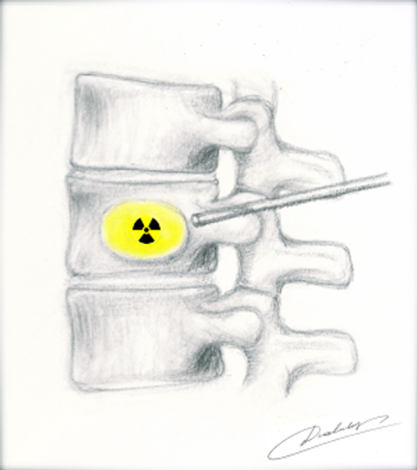
Figure 12 Spine-rad Delivery into vertebrae38
This image shows an illustration of delivery of spine-rad.
The Insignia Hip Stem study is similarly looking at evaluating the safety and effectiveness of Insignia’s Hip Stem used in total hip arthroplasty treatments.37 This study began in February of 2022 and is estimated to be completed in December of 2034, it has 313 participants (Figure 13,14,15).37
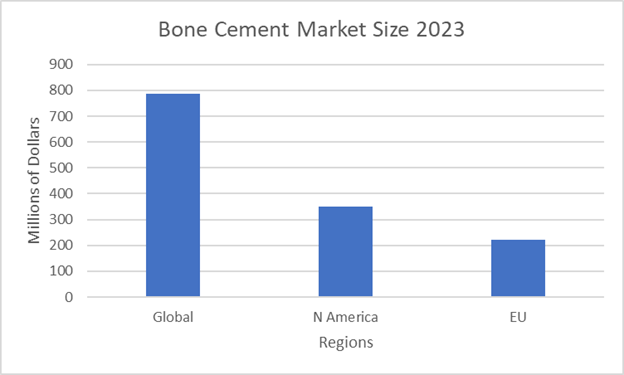
Figure 13 Bone cement regional market data by region 202321
The graph shows the market size in 2023 of various regions.
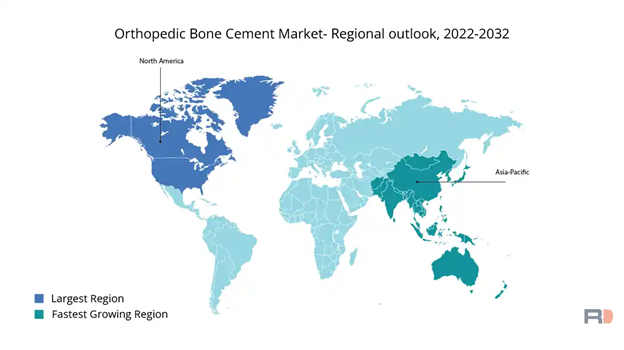
Figure 15 Orthopedic bone cement market 2022-203221
This image shows a world map illustrating forecasted rates of growth from 2022 to 2032.
The bone regeneration study comparing hydroxyapatite vs. hydroxyapatite +hyaluronic acid is evaluating the addition of the acid on bone regeneration after enucleation of mandibular odontogenic cysts.36 Enucleation results in lowered bone density so bone grafts and regeneration can improve the quality of the bone.36 The acid is hypothesized to increase osteogenesis and mineralization.36 This study commenced in October of 2023 and is estimated to be completed by January of 2025, it has 20 participants.36
Future of the industry
The market for bone cement, as discussed earlier, is expected to grow at 3% over the next decade.5 This accounts for increased availability and awareness of current treatments for bone cement40, but the future of the industry is expanding on the clinical uses of bone cement with radiation and tumor treatments.4
Hundreds of thousands of patients are diagnosed every year with cancer that spreads into the spine.38 This painful condition has been looked at by researchers at UC Irvine and they are developing new therapies utilizing radioactive bone cement that is directly injected into the affected bone.38 A study has been conducted involving six sheep, three injected with radioactive cement and three with non-radioactive cement, this was done to show that there were no adverse effects, and the sheep were able to continue walking around without any issue or hindrance (Figure 16).38
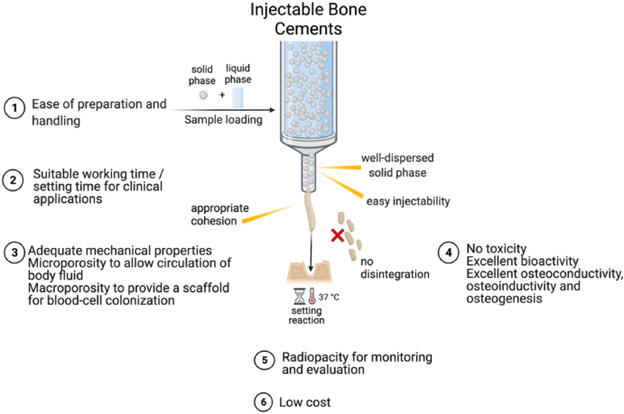
Figure 16 Injectable Bone Cements31
This image shows the properties of an ideal injectable bone cement.
UCI researcher Joyce Kevak has started a company, Bone-Rad, to capitalize on this new frontier of medicine.4 Bone-Rad is developing the product, Spine-Rad, which is being designed to target irradiation to assist in preventing tumor growth and spreading to surrounding bone tissue.39 In the US alone 1.66 million people are diagnosed with cancer each year, over 400,000 of these patients have their cancer metastases into bone and 230,000 of those develop tumors in the spine.39
HA has been recently investigated for controlled drug delivery systems, gene therapies, and bioimaging.40 Controlled drug delivery systems will transport drugs at specific rates and times that should increase the efficiency of the drug.41
Doping HA with metals is being studied to possibly address the disadvantages of HA such as mechanical strength or bioactivity.41 Cu2+, Mg2+, Zn2+, and Ag+ are a few of the specific ions that have been researched to dope HA with (Table 1).41 Doping HA has shown a significant influence on the biomaterial’s mechanical properties.41 Cell viability analyses have shown that co-doping HA, or doping it with 2 or more metals, imparts increased biocompatibility and no cytotoxicity while having improved interfacial bioactivity.41
HA should have a long future as its current use in health care is expected to increase over the next decade, growing consumer awareness and increasing healthcare expenditures.36 It also will grow even more as it reaches into different fields of medicine like cancer treatment.39 HA will continue to grow as researchers employ new methodologies to enhance its properties with metal doping making it even more appealing to healthcare clinical practitioners.40
Recent developments and future considerations
As the market for bone cement continues to grow over the next decade, the demand for improvements and innovations on current offerings will rise, this has led biomedical research to continue pushing bone cements to be more durable with increased endurance.42 Increasing strength and endurance of bone cement could be key in eliminating conditions such as BCIS if the cause of it is determined to be embolus-mediator.17,31
Injectable bone cements, having many desirable qualities, are being used and further developed as they are generally easier to shape, require no manual mixing by the surgeon, are still biocompatible, can be delivered directly to the wound site locally, and can be used as a fixative for an implant that lowers the risk of fracture displacement or loosening.32 Injectables however are less radiopaque, they are still brittle and can be prone to deformation releasing small amounts of cement free into a body, they may rapidly degrade causing local toxicity, and have relatively lower bioactivity causing an increase in fibrous scar tissue formation.32
Bone cement promotes osteogenesis but not to a high degree, it creates high exothermic temperatures when mixed, it does not possess antimicrobial qualities, and it has low mechanical strength.43 These properties limit wider applicable uses of bone cement and overcoming these innate properties requires modifications to the bone cement (Figure 17).43 Most recently, polycitrate-based biomaterials have gained attention in bone repair fields due to their biocompatibility, biomimetic elastic behavior, biodegradability, and overall good tissue repair activity.43 Polycitrate-based biomaterials are not a perfect solution as they still have issues with low mechanical strength, that researchers have been developing ways to overcome by exploring new materials, like poly(citrate-silicon) hybrid polymer (PCS).43 PCS shows it can significantly increase the mechanical strength in bone cements and its osteogenic capacity.43 PCS in addition to improving strength and bone growth also helps by alleviating the exothermic reaction by having a reduced peak temperature.43 PCS also exhibited antioxidant and antimicrobial properties.43 Other developments of reinforcement methods, using tunicate cellulose nanocrystals to modify calcium sulfate bone cements has also shown to increase the mechanical strength and toughness of bone cements.44 Novel approaches like these hold great promise for bone regeneration applications.43,44
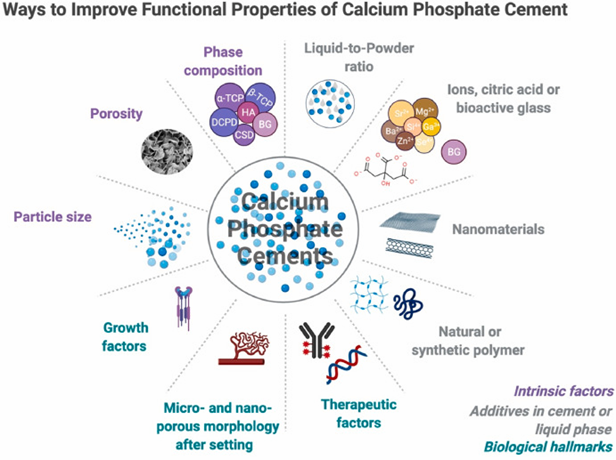
Figure 17 Ways to improve functional properties of calcium phosphate cement31
This image shows a brief overview of the improvement approaches for calcium phosphate cements.
Promoting bone healing is another area of study that is being pursued by researchers to further enhance and grow the industry of bone cements.45 There doesn’t currently exist any biomaterial that can spontaneously regulate the entirety of the bone healing process (Figure 18).45 A study has been performed looking at adding strontium and copper borosilicate glass to help modulate bone healing.45 Adding Sr/Cu-borosilicate glass (BSG) to bone cement has shown to help manage inflammation, angiogenesis, and osteogenesis, all components of the entire bone healing process.45 In vitro and in vivo studies both show successful regeneration of bone, critical bone defect repairs, and enhanced osteogenesis.45
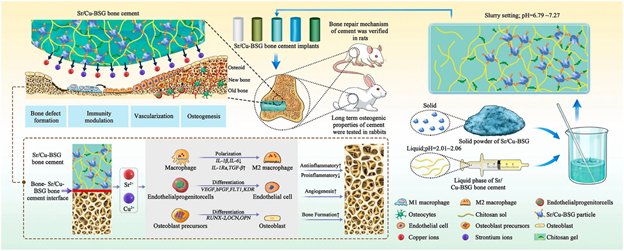
Figure 18 Diagram of bone defect repair43
This image shows a schematic of bone defect repair using Sr/Cu-BSG.
Angiogenesis occurs prior to osteogenesis, as new bone growth requires nutrients and oxygen that vascularization provides to an injured area.45 Cu and Sr ions have been shown to enhance the processes of angiogenesis and osteogenesis.45 The ions help endothelial cells rapidly increase in numbers and the ions enhance osteogenic differentiation of stem cells.45 Excessive inflammation will inhibit the bone healing process and the Sr/Cu-BSG bone cement modulates the polarization of macrophages which significantly reduces inflammation, this has the effect of earlier initiation of the bone healing process, it also degrades at the same rate of the bone growth and healing that it encourages which is an essential aspect of any orthopedic biomaterial.45 The HA that is converted from BSG being chemically similar to bone also helps reduce inflammation as the human body is less likely to view it as a foreign material that it would need to attack.45 Long-term analysis of Sr/Cu BSG, in rabbit femoral studies, supports its biocompatibility by showing no signs of necrosis, inflammation, infection, or tumors.45–48
Bone cements will likely continue to grow as a needed industry due to an overall increasing population and an increase in the aging population.25 Development of enhanced and modified bone cements to overcome their innate disadvantages will be critical in expanding their application into the next generations of medical practitioners and patients.42
Bee-Ling Lim extends her gratitude to Dr. Tawil for the guidance provided during his course taken at UCLA, where he gained substantial knowledge in biocompatibility and bioengineering. Additionally, she wishes to express his thanks to Dr. Tawil for assisting in arranging the publication of this paper.
Authors declare that there is no conflict of interest.
None.

©2024 Bee-Ling, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.