Journal of
eISSN: 2572-8466


Research Article Volume 5 Issue 4
1Department of Processes and Products Development, University of Campinas (Unicamp), Brazil
2Department of Biochemistry and Microbiology, Universidade Estadual Paulista ?Julio de Mesquita Filho? (UNESP), Brazil
3Department of Pharmaceutical Technology, University of Coimbra, Portugal
Correspondence: Jonas Contiero, Department of Biochemistry and Microbiology, Universidade Estadual Paulista (UNESP), Avenida 24A, 1515, Bela Vista, 13506-900, Rio Claro? SP, Brazil, Tel 55 19 35264180
Received: July 18, 2018 | Published: August 22, 2018
Citation: Oliveira RA, Coelho LF, Bernardi NS, et al. Experimental design for optimization of D-lactic acid production using a UV-light selected strain. J Appl Biotechnol Bioeng. 2018;5(4):270-277. DOI: 10.15406/jabb.2018.05.00148
Lactic acid is a molecule with several applications in different industries. Many applications require high isomeric purity, in order to attain the need properties of the final product. L-lactic acid production has already been extensively studied, however, D-lactic acid production remains a challenge since the majority of the strains used in lactic acid production are L producers. Bearing all this in mind, this study presents UV-light as an alternative tool to enhance D-lactic acid production. Different UV-light selected strains from Sporolactobacillus nakayamae subsp. nakayamae were studied in order to obtain high D-lactic acid concentration. Preliminary tests were performed using different carbon and nitrogen sources. Further, an experimental design was used to optimize the medium composition. As result, the selected strain presented a better D-lactic acid production compared to the natural strain, with 97% of isomeric purity. The maximal production of D-lactic acid reached 74.46g/L from clarified sugarcane juice and yeast extract, with a productivity of 3.10g/Lh.
Keywords: D-lactic acid, UV-light, sporolactobacillus nakayamae, experimental design, optimization
Lactic acid (LA) is an organic acid with a hydroxyl and an acid functional group.1,2 It can be produced by humans, plants, animals and microorganisms.2 It has an asymmetric carbon and it naturally occurs as two optical isomers, D- and L-LA, due to the existence of a chiral centre.1,3 It was discovered in 1780 by the experimental chemist Carl Wilhelm Scheele, isolated from sour milk. Currently is known as 2-hydroxypropanoic acid.4
LA chemical production leads to racemic mixtures of D and L isomers.5 This circumstance makes impossible to control the chemical and physical properties of the final product. Besides that, the racemic mixture is not suitable to be used in food, pharmaceutical, and medical industries. The proportion of each isomer confers different physical properties to the final product. It makes the enantiomeric purity a crucial factor for LA industrial production, as in the manufacture of biodegradable plastics and polymers as poly-LA.6
The polymerization of a racemic mixture of L- and D-LA generally leads to the synthesis of poly-DL-LA (PDLLA), which is amorphous. The crystallinity and many other important physical properties such as rate of degradation,3 melting and boiling point 1 are controlled by the ratio of enantiomers D and L used.3,7 Due to the chiral nature of the LA, several different types of polymers can be produced, for example, poly-L-LA (PLLA) is the product resulting from the polymerization of L-LA, which has high crystallinity and high melting point.4,8 The melting temperature of the PLLA may be increased to 50°C by the physical mixture with the polymer PDLA (poly-D-LA) which can form a highly regular stereo-complex with increased crystallinity and thermal stability.9,10
Due to the necessity of enantiomeric purity in the industries for some specific applications, the LA production by fermentation has been more attractive instead of chemical production. Currently, almost all LA in the world is produced by fermentation,11 however, the majority of the microorganisms used and most of the studies in the literature are focused on the L-LA biosynthesis. So far, cost-effective production of L-LA, used for PLLA production, is well established compared to that of D-LA, used for PDLA production, production process.12,13
This finding has led to increased interest in the production of optically pure D-LA because the commercial lactic acid market historically has been dominated by L-LA.10,14
Several microorganisms have been reported to be natural D-LA producers.15–17 Also, genetically engineered microorganisms have been reported to be pure D-LA producers.10,12,14,18–21 However, to the best of our knowledge, it is the first time that UV-light is used as a tool to enhance D-LA production.
Bearing all this in mind, the main objective of this study is to improve the D-LA production with high isomeric purity, using an isolated Sporolactobacillus nakayamae subsp. nakayamae submitted to UV-light mutation. It was also evaluated the effects of different media composition, such as different carbon and nitrogen sources on D-LA production.
Microorganism
The microorganism Sporolactobacillus nakayamae subsp. nakayamae Vini6 is gram-positive, rod-shaped, spore-forming, and a D-LA producer. It was isolated from Desmodium adscendens root.22 The strain was stored in GYP (Glucose-Yeast-Peptone) medium 23 with 20% glycerol (v/v) at - 20°C.
The microorganism was cultivated in GYP culture medium and incubated at 35°C for 24hours. Afterwards, the bacterial suspension was transferred to an Erlenmeyer flask at the same conditions and used as the inoculum. 10% of the workload was inoculated in all fermentation tests.
UV light mutation
nakayamae subsp. nakayamae strain was cultivated in GYP media for 24hours at 35°C, thereafter it was transferred to solid GYP and incubated for 48hours at 35°C. The bacterial colonies were exposed to UV-light (254nm) for 20minutes. After that, NaCl 0.85% was added and the colonies were mixed. The aliquot was used in serial dilutions in solid GYP with CaCO3 1% and incubated at 35°C for 48hours. Selection of colonies was based on rapid growth, as well as on the largest acid production halo. The selected colonies were mixed and incubated to grow and again exposed to UV irradiation for another 20minutes. Three cycles of exposure to UV-light were performed.
Fermentation
All fermentation tests were performed in 40mL Erlenmeyer flasks containing 20mL of medium, at 35°C and 150rpm. Initial pH was adjusted to 7.0 and 60g/L of CaCO3 was added before the inoculum, in order to control the pH during the fermentation time. All tests were performed in triplicate. Samples were stored at -20°C to further analysis after the centrifugation to remove cells and CaCO3.
For each test, LA production (g/L), productivity (Equation 1) and remaining total reducing sugar (RTRS) (Equation 2) were calculated to evaluate the process.
(1)
(2)
Carbon source determination
The fermentation media was composed of 5g/L of sodium acetate, salts solution of GYP media (4% MgSO4; 0.2% MnSO4; 0.2% FeSO4; and 0.2% NaCl), 30g/L of yeast extract and 100g/L of TRS (total reducing sugars) from four different carbon sources: sugarcane juice; clarified sugarcane juice; commercial glucose; commercial sucrose.
Clarified sugarcane juice was prepared using Ca(OH)2, as the method called simple defecation with lime and heating 24: sugarcane juice was limed with Ca(OH)2 to pH 8.0 and placed in the water bath 105°C for 1hour and afterwards filtered, in order to remove the solids.
Nitrogen source determination
The microorganism was incubated in culture media with 5 g/L of sodium acetate, salts solution of GYP media, and 100g/L of TRS (total reducing sugars) from the best carbon source previous determined (item 2.4). Four different nitrogen sources were tested, as shown in Table 1: yeast extract, yeast autolyzed, corn steep liquor (CSL), and pro-floo (cottonseed protein). The statistical analysis was performed using Statistica 7.0 (Statsoft Inc). The results were submitted to F-test with the average results compared by Tukey’s test.
Nitrogen source |
Concentration A |
Concentration B |
Yeast extract (g/L) |
15 |
30 |
Yeast autolyzed (g/L) |
12.75 |
25.5 |
CSL (mL/L) |
36.75 |
73.5 |
Pro-floo (g/L) |
15.47 |
30.94 |
Table 1 Nitrogen source concentration
The amount of each nitrogen source corresponds to the same amount of nitrogen contained in 15g/L and 30g/L of yeast extract. Concentration A corresponds to 1.725g of nitrogen/L; Concentration B corresponds to 3.45g of nitrogen/L.
Experimental design
In order to evaluate the influence of TRS, nitrogen source, sodium acetate and GYP salts solution for D-LA production, it was performed a factorial central composite design with four replicates at the central point, totalizing 28 experiments. Table 2 shows concentrations and levels of the 4 independent variables analysed. The Statistic 7.0 (Statsoft Inc.) was used to analyse the experimental design. The confidence level for the regression test was fixed at 95%. The results were submitted to the F-test and the average results compared by Tukey’s test.
Variables |
Codes |
Levels |
||||
-2 |
-1 |
0 |
1 |
2 |
||
TRS (g/L) |
X1 |
0 |
50 |
100 |
150 |
200 |
Yeast extract (g/L) |
X2 |
0 |
15 |
30 |
45 |
60 |
Sodium acetate (g/L) |
X3 |
0 |
5 |
10 |
15 |
20 |
Salts of GYP (mL/L) |
X4 |
0 |
2.5 |
5 |
7.5 |
10 |
Table 2 Real values of the coded independent variables
The association between the factors and response was modeled using polynomial equation given by Equation 3, in which: X1, X2, X3 and X4 are the independent coded variables, β0, β1, β2, β3, β4, β12, β13, β14, β23, β24 and β34 are the regression coefficients, and Y is the response function.
(3)
Analytical methodology
For LA and sugars quantification it was used a high-performance liquid chromatography (HPLC) (Shimadzu, Prominence series) equipped with an ultra-violet detector at 210nm and refractive index detector. The column used was Rezex ROA (300 x 7.8mm) from Phenomenex, eluted with 5mM of H2SO4 as the mobile phase. The flow was 0.6mL/min and the injection volume was 5 µL. The temperature was set at 65°C.
In order to determine the concentration and the isomeric purity of LA, HPLC was equipped with a Chirex 3126 column (150 x 4.6mm) from Phenomenex and a UV detector. The flow was adjusted to 1mL/min using 1mM of CuSO4 as the mobile phase and the temperature was fixed at 26°C.
The microorganism S. nakayamae subsp. nakayamae Vini6 is a natural producer of D-LA with a yield up to 50%. The natural strain was able to produce 12.77g/L of LA from 20g/L glucose, with 92.01% of D-LA purity.
The natural strain was exposed to UV-light for 20minutes. After the first UV-light exposure originated the selected strain called Vini6 2x (second generation). This new strain was exposed to UV-light and generated the strain Vini6 3x (third generation). The UV-light cycle was repeated one more time and generated Vini6 4x (fourth generation), Vini6 4x2 (fourth generation colony 2), Vini6 4x3 (fourth generation colony 3), and Vini6 4x4 (fourth generation colony 4). In total, 6 strains were selected from UV-light exposure, which are depicted in Table 3. All selected strains presented a better D-LA production when compared to the natural strain. The highest LA production obtained from 100g/L of glucose was 88.98 g/L using the selected strain S. nakayamae Vini6 2x, which was chosen as the strain to be used in the next tests.
Strain |
D(-) lactic acid (g/L) |
S. nakayamae Vini6 natural strain |
60.64 |
S. nakayamae Vini6 2x |
88.98 |
S. nakayamae Vini6 3x |
76.9 |
S. nakayamae Vini6 4x |
59.85 |
S. nakayamae Vini6 4x2 |
81.95 |
S. nakayamae Vini6 4x3 |
79 |
S. nakayamae Vini6 4x4 |
79.68 |
Table 3 D(-) acid lactic production by 6 strains submitted to UV mutation from 100g/L of glucose
After the strain selection, D-LA production was tested using different carbon sources: sugarcane juice, clarified sugarcane juice, commercial glucose, and commercial sucrose. The highest D-LA production occurred in the presence of commercial sucrose as a carbon source, both after 24 and 48hours (56.61 and 58.98g/L, respectively). However, commercial sucrose has high market prices, which justifies its replacement by a more economically viable source of carbon. Therefore, it is noted the clarified sugarcane juice has a slightly higher production of D-LA (53.80g/L) when compared to sugarcane juice (48.18g/L). The clarification was performed in order to precipitate the largest possible amount of impurities, resulting in an improvement of the microorganism performance. The process did not affect the sucrose content, but pectin, albumin, mucilage, starch, fibres, waxes, proteins, pigments, and other components precipitated and were removed with a simple filtration.24 Front of the results clarified sugarcane juice was chosen as the carbon source to D-LA production.
The next step was to test different nitrogen sources to be used for D-LA production: yeast extract, yeast autolyzed, CSL, and pro-floo. The results of the nitrogen source influence in production, productivity and yield of D-LA are shown in Table 4. The highest D-LA production was 67.89g/L using yeast extract on the concentration B, after 24hours of fermentation. Remaining TRS also presented the best result using yeast extract (6.15g/L) on the concentration B. Only when LA yield is considered, corn steep liquor presented the best result, reaching 0.75g/g after 48hours of fermentation, also on concentration B. However, yeast extract also presented a good yield (0.58g/g), being chosen as the nitrogen source to be used for D-LA production.
Nitrogen source |
Concentration A |
Concentration B |
Production - 24 hours |
||
Yeast extract |
50.82 Ba |
67.89 Aa |
Yeast autolyzed |
3.83 Ac |
5.02 Ac |
Corn steep liquor |
37.60 Ab |
39.13 Ab |
Pro-floo |
0.37 Ac |
0.48 Ac |
Production - 48 hours |
||
Yeast extract |
50.92 Aa |
54.83 Aa |
Yeast autolyzed |
1.24 Ab |
1.82 Ab |
Corn steep liquor |
45.58 Ba |
58.67 Aa |
Pro-floo |
0.13 Ab |
0.47 Ab |
Remaining TRS - 48 hours |
||
Yeast extract |
12.61 Ab |
6.15 Ab |
Yeast autolyzed |
55.89 Aa |
62.00 Aa |
Corn steep liquor |
17.56 Ab |
19.70 Ab |
Pro-floo |
57.55 Ba |
68.99 Aa |
Yield - 48 hours |
||
Yeast extract |
0.58 Aa |
0.58 Ab |
Yeast autolyzed |
0.03 Ab |
0.05 Ac |
Corn steep liquor |
0.55 Ba |
0.75 Aa |
Pro-floo |
0.00 Ab |
0.02 Ac |
Table 4 D(-) lactic acid production (g/L) after 24 and 48 hours of fermentation, remaining TRS (g/L), and yield (g/g), according to the nitrogen source used
An average value followed by different letters in vertical (lowercase) and horizontal (uppercase) differ at 5% according to Tukey’s test.
Based on the preliminary tests, clarified sugarcane juice and yeast extract were used in the central composite design in order to optimize the media composition for D-LA production. Table 5 shows the variables and the real concentrations of each component: TRS from clarified sugarcane juice (X1), yeast extract (X2), sodium acetate (X3), and salts solution from GYP (X4), as well as the average results obtained from a triplicate of D-LA production, productivity and remaining TRS after 24hours of fermentation.
Test |
Concentration |
Production (g/L) |
Productivity (g/Lh) |
Remaining TRS (g/L) |
|||
TRS |
Yeast extract |
Acetate |
GYP salts |
||||
1 |
50 |
15 |
5 |
2.5 |
1.37 |
0.06 |
46.58 |
2 |
50 |
15 |
5 |
7.5 |
1.77 |
0.07 |
48.51 |
3 |
50 |
15 |
15 |
2.5 |
7.48 |
0.31 |
36.97 |
4 |
50 |
15 |
15 |
7.5 |
6.98 |
0.29 |
33.65 |
5 |
50 |
45 |
5 |
2.5 |
4.92 |
0.2 |
33.94 |
6 |
50 |
45 |
5 |
7.5 |
25.88 |
1.08 |
5.4 |
7 |
50 |
45 |
15 |
2.5 |
36.53 |
1.52 |
2.83 |
8 |
50 |
45 |
15 |
7.5 |
6.34 |
0.26 |
30.58 |
9 |
150 |
15 |
5 |
2.5 |
3.36 |
0.14 |
138.58 |
10 |
150 |
15 |
5 |
7.5 |
27.89 |
1.16 |
96.12 |
11 |
150 |
15 |
15 |
2.5 |
8.92 |
0.37 |
132.14 |
12 |
150 |
15 |
15 |
7.5 |
3.21 |
0.13 |
132.34 |
13 |
150 |
45 |
5 |
2.5 |
73.49 |
3.06 |
28.91 |
14 |
150 |
45 |
5 |
7.5 |
74.46 |
3.1 |
31.06 |
15 |
150 |
45 |
15 |
2.5 |
58.51 |
2.44 |
45.79 |
16 |
150 |
45 |
15 |
7.5 |
55.75 |
2.32 |
54.55 |
17 |
0 |
30 |
10 |
5 |
3.93 |
0.16 |
0 |
18 |
200 |
30 |
10 |
5 |
48.48 |
2.02 |
97.91 |
19 |
100 |
0 |
10 |
5 |
1.4 |
0.06 |
100 |
20 |
100 |
60 |
10 |
5 |
51.67 |
2.15 |
32.34 |
21 |
100 |
30 |
0 |
5 |
63.39 |
2.64 |
3.16 |
22 |
100 |
30 |
20 |
5 |
31.89 |
1.33 |
26.55 |
23 |
100 |
30 |
10 |
0 |
64.27 |
2.68 |
4.92 |
24 |
100 |
30 |
10 |
10 |
61.12 |
2.55 |
3.64 |
25 |
100 |
30 |
10 |
5 |
66.58 |
2.77 |
4.15 |
26 |
100 |
30 |
10 |
5 |
64.27 |
2.68 |
3.7 |
27 |
100 |
30 |
10 |
5 |
61.05 |
2.54 |
3.93 |
28 |
100 |
30 |
10 |
5 |
61.57 |
2.57 |
3.58 |
Table 5 Central composite design (real values), and the results after 24 of fermentation
The highest D-LA production was obtained in test 14, with 74.46g/L of LA produced from 150g/L of TRS, 45g/L of yeast extract, 5g/L of sodium acetate, and 7.5mL/L of GYP salts solution, as shown in Table 5. On these conditions, productivity and remaining TRS were 3.10g/Lh and 31.06g/L. D-LA isomer purity presented an average of 97%±2% between the 28 tests, showing that this microorganism may be suitable for an industrial process for D-LA production.
The application of multiple regression analysis for each response from Table 5 resulted in the regression Equations 4, 5 and 6. Equation 4 represents the regression model for LA production (LA), Equation 5 for productivity (P), and Equation 6 for remaining TRS (RTRS). X1, X2, X3 and X4 are the coded values of the test variables TRS, yeast extract, sodium acetate and GYP salts, respectively.
(4)
(5)
(6)
The identification of significant parameters was performed through hypothesis test using t-Student statistic method. The probability was used as a tool to verify the significance of each coefficient. The maximum probability of error in the test was 5%, thus the parameters with the value p<0.05 were considered significant.
The main variables affecting production, productivity and remaining TRS are X1 (TRS) and X2 (yeast extract). Based on these results, it was possible to imply that acetate (X3) and salts solution from GYP (X4) were not essential for D-LA production using S. nakayamae Vini6 2x.
Fisher-test was used to analyse the statistical significance of the Equations 4, 5 and 6 through ANOVA, as shown in Table 6.
Production |
|||||
Source of variation |
Sum of Squares |
Degrees of Freedom |
Mean Square |
Fcalculated |
P |
Regression |
19782.06 |
14 |
1.41 |
5.32 |
0.00232 |
Residues |
3453.05 |
13 |
265.62 |
||
Lack of fit |
3432.77 |
10 |
343.27 |
||
Pure error |
20.28 |
3 |
6.76 |
||
Total |
23235.11 |
27 |
|||
R2 = 0.85; R = 0.92 |
|||||
Productivity |
|||||
Regression |
3.18 |
14 |
2.27 |
7.82 |
0.000333 |
Residues |
378.25 |
13 |
0.29 |
||
Lack of fit |
374.91 |
10 |
0.37 |
||
Pure error |
0.03 |
3 |
0.01 |
||
Total |
3.56 |
27 |
|||
R2 = 0.89; R = 0.94 |
|||||
Remaining TRS |
|||||
Regression |
35426.3 |
14 |
2.53 |
4.94 |
0.00329 |
Residues |
6657.28 |
13 |
512.1 |
||
Lack of fit |
6657.11 |
10 |
665.71 |
||
Pure error |
0.18 |
3 |
0.06 |
||
Total |
42083.58 |
27 |
|||
R2 = 0.84; R = 0.92 |
|||||
Table 6 ANOVA table for the responses with 95 % of confidence level
For Equation 4, F14,13 calculated (5.32) was higher than F14,13 tabulated (2.55) with 95% of confidence level, showing that the model is significant and adequately explains experimental data variation for LA production. The good fit of the model was verified by the determination coefficient (R2) and multiple correlation co-efficient (R). In this case, the value of R2 indicates that 85% of the variability in the data was explained by the mathematical model. The high R-value (0.92) shows a high degree of agreement between the experimental data and predicted values (Table 6). Based on the mathematical model it was constructed the response surface for LA production in function of TRS (X1) and yeast extract (X2). To achieve high lactic acid concentrations, Figure 1A shows that the optimum concentration of TRS should be between 100 and 200g/L and between 30 and 60g/L for yeast extract.
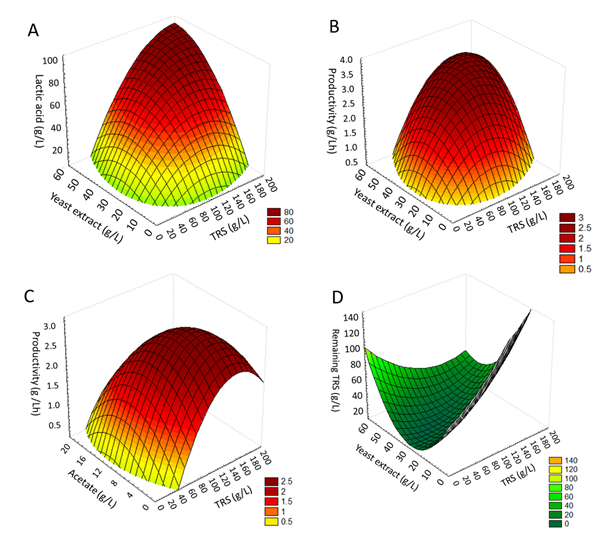
Figure 1 Response surface of (A) lactic acid production in function of TRS (X1) and yeast extract (X2); (B) lactic acid productivity in function of TRS (X1) and yeast extract (X2); (C) lactic acid productivity in function of TRS (X1) and acetate (X3); and (D) remaining TRS in function of TRS (X1) and yeast extract (X2).
Considering the productivity (Equation 5) Fisher-test also confirmed that the mathematical model was predictive. F14,13 calculated (7.82) was higher than F14,13 tabulated (2.55) with 95% of confidence level, showing that the model is significant. In this case, R2 indicated that 89% of the variability in the data was explained by the model. The high R-value (0.94) shows a high degree of agreement between the experimental data and predicted values (Table 6). Thus, it was possible to construct the response surface for LA productivity in function of TRS (X1) and yeast extract (X2) as shown in Figure 1B; and in function of TRS (X1) and acetate (X3) as shown in Figure 1C. According to Figures 1B and 1C, better productivities can be achieved when the concentrations of TRS are between 120 and 180g/L of TRS, yeast extract is between 35 and 55g/ L, and sodium acetate is between 4 and 10g/L.
According to Equation 6, F14,13 calculated (4.94) was higher than F14,13 tabulated (2.55) with 95% of confidence level, showing that the mathematical model was significant and adequately explained the experimental data variation for LA yield. In this case, the value of R2 indicated that 84% of the variability in the data was explained by the model. The high R-value (0.92) also shows a high degree of agreement between the experimental data and predicted values (Table 6). Based on these results, Figure 1D shows that in order to obtain low amounts of remaining TRS, initial TRS concentrations should be around 100g/L and yeast extract should be 30g/L.
In all cases, residual distribution responses were random around zero with no tendency. The observed values were close to those predicted, leading to a steady trend and a normal distribution.
In general, it was observed that high TRS concentration (up to 150g/L) leads to a decrease in the productivity and a higher amount of remaining TRS. In this context, the best media composition for S. nakayamae Vini6 2x should be composed by 150g/L of TRS from clarified sugarcane juice and 35g/L of yeast extract. 4g/L of sodium acetate could also be used as a complementary nutrient in order to enhance the productivity.
Several microorganisms have been reported to be D-LA producers, such as Lactobacillus delbrueckii15 Lactobacillus coryniformis17 and Lactobacillus plantarum.10,14,18,19 Several Sporolactobacillus sp. strains have also been reported to be D-LA producers.16,22,25–33 Even though, D-LA production is still a challenge for the industry,12,13 once the majority of good LA producers have key-enzymes to L-LA production.
In this sense, many studies report the use of metabolic engineering tools to obtain higher D-LA production.10,12,14,18–21,29,33,34 Instead of using metabolic engineering tools, this study presents a UV-light mutation approach to improve the D-LA production by S. nakayamae nakayamae. This kind of approach is widely used for mutation and selection of microorganisms in order to improve their capacity of producing biologically active substances.35,36 The use of UV-selection mechanism is an effective mutagenesis tool towards improving lactic acid production in different strains, as demonstrated for Lactobacillus lactis BME5-18 with a productivity 46% higher than the parent strain 37 and Rhizopus oryzae R1021 with an improvement of 52% of LA production.36
Clarified sugarcane juice and yeast extract were the best carbon and nitrogen sources between the ones evaluated for D-LA production by S. nakayamae Vini6 2x.
Yeast extract had been chosen once yeast extract makes it possible to have a faster D-LA production. A study using S. inulinus detected that the free amino acids are the limiting factor for the lactic acid production 30. It turned out that this is the reason why yeast extract is a good nitrogen source for this microorganism. Furthermore, Brazil has a high potential of using the remaining yeast from the ethanol industry to produce yeast extract with low costs. As highlighted by Pleissner et al.,38 the costs of biotechnological inputs are region dependent. For instance, in the case presented here, yeast extract can be an important input if the LA production could be done close to an ethanol and yeast production plant, in a biorefinery concept. Ethanol fermentation as it is done in Brazilian distilleries generates an excess of 20kg of dried yeast per 1000L of produced ethanol39 that can be converted to a low-cost yeast extract. This process is already cost-effective in Brazilian scenario.40 As an example, a Sugarcane 1st Generation Biorefinery would process sugarcane to sucrose, ethanol, yeast products and LA. The remaining bagasse can be still used to provide steam and electricity to sustain processes and the excess of electricity available can be even exported to the grid.
Regarding the optimization process, the main components affecting D-LA production by S. nakayamae Vini6 2x are clarified sugarcane juice and yeast extract. Higher D-LA concentrations can be obtained using 150 g/L of TRS from clarified sugarcane juice and 35g/L of yeast extract. 4g/L of sodium acetate can also be used as a complementary nutrient in order to enhance the productivity. Zheng et al.32 proved that the presence of sodium ions in the early growth phase of S. inulinus improve the productivity of D-LA by the key-enzyme phosphofructokinase. Besides, sodium acetate is also known as an efficient medium component to prevent the contamination of the process by fungi.41
S. nakayamae Vini6 2x is a useful strain to produce D-LA with high purity (≈97%). UV-light is a suitable tool for increasing the LA production, as well as keeping a high isomeric purity. The use of central composite design was a useful tool to optimize the production process of D-LA. Further, the obtained results are very promising for scaling-up the fermentation process. As next steps, it is intended to scale-up the fermentation process in order to obtain higher D-LA production and productivity.
None.
Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (2012/21679-9)
Author declares that there is no conflict of interest.

©2018 Oliveira, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.