Journal of
eISSN: 2572-8466


Research Article Volume 1 Issue 2
Faculty of Lifesciences and Biotechnology, South Asian University, India
Correspondence: Gobinath Rajagopalan, Faculty of Lifesciences and Biotechnology, India
Received: July 16, 2016 | Published: November 16, 2016
Citation: Rajagopalan G. Effect of nitrogen salts on butanol and hydrogen production from xylan by using clostridium strain BOH3 . J Appl Biotechnol Bioeng. 2016;1(2):44-51. DOI: 10.15406/jabb.2016.01.00008
Clostridium strain BOH3 ferments 10g/l of xylan in reinforced clostridial medium (RCM) and produces butanol and hydrogen. However, increasing xylan concentration above 10g/l does not lead to higher concentrations of butanol and hydrogen. In this study, it shows that addition of ammonium sulfate to RCM can enhance cell density and the production of butanol and hydrogen. Based on response surface methodology (RSM), an optimized culture medium (OCM) was developed with 58.4g/l of xylan and 11.8g/l of ammonium sulfate to maximize butanol (12.15g/l) and hydrogen (3.29l/l) production. From RSM, butanol production depends on xylan concentration, whereas hydrogen production depends on ammonium sulfate concentration. Clostridium BOH3 in OCM also expresses 7.15U/ml of xylanase during fermentation, and the xylanase can be purified and recycled to further enhance the production of butanol and hydrogen.
Keywords: Clostridium spp, ABE fermentation, consolidated bioprocess, biohydrogen, xylanase
Fossil fuels are regarded as limited resources. Globally, every year over 11 billion tonnes of fossil fuels are consumed and the demand is expected to increase yearly at the rate of 1.7%.1,2 Moreover, continuous consumption of fossil fuels increases the emissions of CO2, nitrogen monoxide and sulphur dioxide, which may lead to global warming or climate changes.3 To meet global energy demand and minimize environmental impact, several alternative energy sources have been proposed. Among them, a variety of liquid fuels including methanol, ethanol, butanol and biodiesel and gaseous fuels such as hydrogen and methane can be produced from renewable biomass.4,5
Butanol is an ideal liquid fuel because it has high energy density value (29 MJ/l) and it can be blended in any ratios with gasoline and diesel. It has low vapour pressure (0.53 kPa) such that it can be transported easily using the existing pipelines. It is also less hygroscopic and less corrosive to pipelines and engines. Moreover, its low water solubility reduces the potential risk of ground water contamination.3,6,7 Similarly, hydrogen is an ideal gaseous fuel due to the following advantages. First, its energy content is much higher (143 GJ/ton) than other gaseous fuels. Secondly, it is a clean and carbon-free fuel, because upon oxidation it produces only water without CO2. Finally, it has the potential to be converted to electricity directly by using fuel cells.8,9 Interestingly, both butanol and hydrogen can be produced from anaerobic Clostridial fermentation.
In Clostridial fermentation, hydrogen and butanol are produced from two different phases. During the active growth phase, Clostridium produces hydrogen and volatile fatty acids (VFAs) predominantly acetic and butyric acids (acidogenesis phase) and later at stationary phase these acids can be reassimilated and subsequently produces solvents (solventogenesis phase) including acetone, butanol and ethanol (ABE).7-10 In principle, Clostridium favours the acidogenesis phase for two purposes. Firstly, it can acquire more ATPs. Secondly, cells can maintain redox equilibrium by utilizing the redox potentials formed in glycolysis. Meanwhile, excess reducing equivalents presence in the cell transfer the electrons to [FeFe] hydrogenase that drives the evolution of hydrogen.11,12 On the other side, when the acids concentration exceeds, the survival of Clostridium cell becomes very difficult at low pH. Thus, Clostridium switches to solventogenesis phase to reassimilate certain amount of acids in the medium to butanol.11,13-15
There are several feed stocks considered for butanol and hydrogen production from Clostridial fermentation. Starchy materials (maize, wheat, millet, rye, etc) or sugars (glucose, xylose and molasses) are conventional substrates. However, these substrates are also food sources and tend to be more expensive than lignocellulosic materials such as cellulose and xylan.7,15 Because lignocelluloses are abundant and low-cost, they are more suitable for the production butanol and hydrogen. Normally cellulosic biomass contains 20-40% of hemicelluloses and xylan is the major hemicellulose.5 Utilizing xylan as a feed stock for Clostridial fermentation would provide a sustainable and economically viable bioprocess for butanol and hydrogen production.3,16 To utilize xylan or xylan related substrates for growth and butanol production, Clostridium requires simultaneous expression of saccharolytic key enzyme xylanase. Unfortunately, most of the solventogenic Clostridium strains do not express xylanase. Hence, xylan cannot be used as a sole carbon source.17,18 For instance, when C. acteobutylicum ATCC39236 was cultured in a xylan-based medium, it showed a longer lag phase up to 240h due to inadequate xylanase expression.18 To minimise the lag time and also to support the initial growth of C. acteobutylicum P260, the medium was amended with 5g/l of xylose (or glucose) and xylanase.17 It is also noticed that in most cases xylan was autoclaved (121°C for 15-30min), but solventogenic Clostridium strains still could not effectively ferment autoclaved xylan. Subsequently, to enhance the utilization of xylan or xylan enriched substrates by Clostridium strains, a few pretreatment approaches based on using moist heat (160°C for 2h), acid (0.5%v/v sulphuric acid at 121°C for 1h), aqueous monoethanolamine (186°C for 3h) and adding commercial xylanase (so-called simultaneous saccharification and fermentation) were suggested in the literature.17-20 On the other side, a few solventogenic Clostridial strains including Clostridium strains IBUN 22A and 62B, C. beijerinckii (LU series 1-4), C. acetobutylicum (LU1 and 2), C. butyricum LU1 and C. bifermentans LU1 expressed xylanase (0.5 to 4.2U/ml) when they cultured in xylan medium. But they produced 1.8 to 5.1g/l of solvents (ABE) from only xylose or glucose supplemented medium and their potential on direct fermentation of xylan into solvent production were not demonstrated.21,22 Similarly, a xylanase expressing saccharolytic Clostridium strain X53 produced only hydrogen (1.25l/l) and no solvents from autoclaved xylan (10g/l).23
Despite butanol and hydrogen can be produced simultaneously in ABE fermentation, it is noticed that most research groups only focused on either butanol or hydrogen. In other words, they channelled most of the electron and carbon flow into a specific metabolic pathway to maximize the target product formation and avoided bifurcation of carbon energy into two pathways. For instance, to enhance hydrogen production, carbon source was limited (<10g/l) to minimise the lag time and the pH was maintained close to pH7 to accelerate acid and hydrogen formation without solvents.11,23 Similarly, to enhance butanol production, partial pressure of hydrogen in the head space of fermentation was adjusted to between 100 and 250kPa whereas medium pH was maintained in the acidic region (pH 5.5-4.5). This strategy can lead to much lower hydrogen evolution and high butanol production.11,24 However, since both hydrogen and butanol can be used as fuels, it would be interesting to maximize the production of both hydrogen and butanol simultaneously.
The common issues identified in butanol and hydrogen production from xylan substrates by Clostridium strain include lack of xylanase expression, acid intolerance and inefficient enzymes which involve in butanol formation. Recently, Clostridium strain BOH3 is reported to produce high butanol and hydrogen from various substrates including glucose, xylose and xylan. Strain BOH3 naturally possess high level of solvent (>220mM) and acid (>190mM) tolerance.25 It also expresses highly efficient (4.02±0.07 μmol/(mg protein. min)) and stable butanol dehydrogenase which ensures high butanol formation. Additionally, strain BOH3 shows strong xylanolytic activity (2.08±0.03U/ml). Subsequently, it grows in xylan (10g/l) supplemented medium and produces butanol (2.1±0.05g/l) and hydrogen (0.55±0.02l/l).26-28 When the xylan concentration is increased from 10 to 30g/l, the cell density increased but the xylanase activity, butanol and hydrogen production did not increase proportionally. Presumably, some components in the medium are insufficient and that lead to lower butanol and hydrogen production. In batch fermentation, Clostridium strain requires several nutrient components for its growth and solvent production. In addition to carbon source, nitrogen source is also found to play a major role in several cellular functions including biomass generation, intracellular biomolecules synthesis, metabolic regulation and also solvent production.29 In ABE fermentation, a decreased nitrogen source in the medium hampers sufficient biomass generation and effective utilization of carbon source. Noticeably, some Clostridium strains failed to switch to solvent production in a low nitrogen-containing medium 24,29. Therefore, it has been proposed that a specific combination of carbon and nitrogen (C/N ratio) source in medium functions synergistically in Clostridial fermentation, and favours higher production of butanol and hydrogen.11,15 In this study, a specific combination of xylan substrate and a nitrogen source (C/N ratio) is designed based on response surface methodology (RSM) to enhance the production of butanol and hydrogen directly from xylan. Further investigation shows that the consolidated bioprocess developed can effectively ferment high concentration of xylan (~60g/l) and produces butanol and hydrogen which are almost equivalent to the productions from xylose supported medium.
Materials
Ammonium chloride, ammonium sulfate, sodium nitrate, potassium nitrate, urea, beechwood xylan and xylose were purchased from Sigma-Aldrich (U.S.A). Reinforced clostridial medium, yeast extract, peptone and tryptone were procured from Difco (U.S.A).
Fermentation
The microorganism used in this study was Clostridium strain BOH3 [25,28]. A commercial reinforced clostridial medium (RCM) was used for culturing strain BOH3. Details of medium compositions and preparation can be found elsewhere.26 Briefly, 20ml of RCM medium (1.5×concentrated) was dispensed into a 120ml serum bottle while the bottle was purging with N2 to remove O2. Later, the bottle was sealed with a rubber septum and an aluminium cap to provide an anaerobic condition. Subsequently, the bottle containing RCM medium was autoclaved at 121°C for 20min. Xylan was used as a carbon source. For the selection of nitrogen source (5g/l), a few organic nitrogen sources (yeast extract, peptone and tryptone) and some inorganic nitrogen salts (ammonium chloride, ammonium sulfate, sodium nitrate, potassium nitrate and urea) were individually added to RCM containing 30 g/l of xylan. After inoculation (10%v/v), the bottle were incubated at 35°C under constant shaking (150rpm). Samples were drawn periodically (12h) and centrifuged at 10,000rpm for 15min and the clear supernatant was used to analyse xylanase activity and other liquid products formed. In parallel, to analyse gaseous products the samples were drawn with a gas-tight syringe every 6h from the head space of fermentation bottle.
Xylanase assay and purification
One millilitre of reaction mixture containing 0.5% (w/v) of beechwood xylan and 100μl of the enzyme sample dissolved in sodium acetate buffer (50mM, pH 5) was incubated at 35°C for 15min and the amount of reducing sugars released was measured by using DNS method. One unit of enzyme activity was defined as the amount of enzyme that liberated 1μmol of reducing sugar per minute. Protein concentration was determined by using Bradford assay according to manufacturer’s (Bio-Rad) instructions. In some cases, xylanase was purified from the cell-free supernatant by a fast-flow anion exchange column Q-Sepharose (GE Healthcare) using FPLC.27
Response surface methodology (RSM)
The experimental design was a 22 full factorial central composite rotatable experimental plan with two medium components, i.e. xylan and ammonium sulfate. The experimental plan consisted of 13 trials including five centre points and the value of the dependent response was the mean of three independent experiments. The response variable obtained using RSM was fitted to the second order polynomial equation
(1)
where
is the predicted response,
are input variables which influence the response variable ![]() ;
is the offset term;
is the ith linear coefficient;
is the ith quadratic coefficient and
is the ijth interaction coefficient. The second order polynomial coefficients were calculated and analyzed using the ‘Design Expert’ (Version 8.0, Stat-Ease Inc., Minneapolis, USA) statistical software package. Statistical analysis of the model was performed by the analysis of variance (ANOVA).28 All runs in central composite plan were performed in triplicates and the average values obtained from nine independent experiments were presented in results.
;
is the offset term;
is the ith linear coefficient;
is the ith quadratic coefficient and
is the ijth interaction coefficient. The second order polynomial coefficients were calculated and analyzed using the ‘Design Expert’ (Version 8.0, Stat-Ease Inc., Minneapolis, USA) statistical software package. Statistical analysis of the model was performed by the analysis of variance (ANOVA).28 All runs in central composite plan were performed in triplicates and the average values obtained from nine independent experiments were presented in results.
Butanol and hydrogen production
Beechwood xylan (30 to 70g/l) and ammonium sulfate (5 to 15g/l) supplemented in RCM was fermented by strain BOH3 for butanol and hydrogen production. To investigate the effect of xylanase on production of butanol and hydrogen, a certain amount (0.5 to 2.5U/ml) of commercially available xylanase from Thermomyces lanuginosus (Sigma, USA) was added to optimized culture medium (OCM) at the beginning of fermentation. OCM was composed of 58.4g/l of beechwood xylan and 11.8g/l of ammonium sulfate supplemented in RCM. Later, instead of adding commercial xylanase, the in-house produced xylanase (2.5-3U/ml) from a previous batch of BOH3 fermentation was added to OCM and the fermentation was conducted anaerobically at 35°C for 7-10 days under the agitation speed of 150rpm. Samples were drawn at every 6-12h intervals to estimate the gaseous and liquid products.
Product analysis
Liquid samples were analyzed by using gas chromatography (GC; model 7890A; Agilent technologies, USA). The GC is equipped with a Durabond (DB)-WAXetr column (30 m × 0.25 mm × 0.25 µm, model 123-7334; J&W, USA) and a flame ionization detector (FID). The oven temperature was set at 60°C for 2min, then it was increased with a ramp of 10°C/min to 230°C and held for 2min. Helium was used as carrier gas with a flow rate of 2.5 ml/min. Acetone, butanol, ethanol, acetic and butyric acids (Sigma, U.S.A) were added into liquid samples as internal standards for qualitative analysis. Based on increase in peak areas, the specific component was identified in the liquid samples. For quantitative analysis of these products, the standard curves were obtained by running known concentrations (0.5 to 25 g/l) of standard solutions using the same method. On the other side, gaseous samples were analysed for hydrogen production with another GC (model GC-17A; Shimadzu, Japan) equipped with a thermal conductivity detector (TCD) and a Supelco 80/100, Porapak-N column, (2 m × 1/8 inch stainless steel column). The oven temperature was kept constant at 110°C for 2.3 min and argon (15 ml/min) was used as the carrier gas. One hundred microlitre of gas was drawn from the head space of fermentation bottle by using a gas-tight syringe and immediately injected into GC-TCD to begin the analysis. For every fermentation bottle, this procedure was repeated for three times and the average was computed for hydrogen production. Standard gaseous mixtures consisting of hydrogen (5%), nitrogen (60%), carbon dioxide (15%), carbon monoxide (15%) and methane (5%) at known proportions were used to obtain a calibration curve.
Elemental analysis
Medium components including xylan and RCM were analysed for carbon and nitrogen (%w/w) by elementary analyzer (vario MACRO cube, Elementar) using the sophisticated facilities from Chemical, Molecular and Materials Analytical Centre, National University of Singapore, Singapore.
Fermentation of xylan by strain BOH3
When Clostridium strain BOH3 cultured in RCM with 20 and 30g/l of xylan, the maximum cell density (OD600) was observed after 12h of fermentation, they were estimated 3.25 and 3.80 respectively. After 5 days of fermentation, the RCM supplemented with 20-30g/l of xylan was supported to produce 2.28-2.55g/l of butanol and 0.70-0.81l/l of hydrogen. When culturing strain BOH3 in RCM with 10g/l of xylan, the cell density was 2.19 (OD600) and the butanol as well as hydrogen production were estimated 2.1g/l and 0.55 l/l, respectively.27 This phenomenon implies that increasing cell density does not guarantee higher butanol and hydrogen concentrations in ABE fermentation. Considering the vital role of nitrogen source in Clostridial fermentation, we hypothesize that insufficient nitrogen sources is the main cause for the incomplete utilization of xylan. Therefore, a few organic and inorganic nitrogen sources were added to enhance the production of butanol and hydrogen. Table 1 shows the effect of nitrogen sources on butanol and hydrogen production from 30g/l of xylan. Among the organic sources tested, yeast extract supports high cell density (4.28) and butanol (3.15g/l) production, but the hydrogen concentration does not increase significantly. Meanwhile, adding ammonium sulfate leads to higher butanol (3.32g/l) and hydrogen (0.90l/l) production. Thus, ammonium sulfate is the most preferable nitrogen source for strain BOH3 to produce butanol and hydrogen simultaneously from xylan. In the literature, ammonium salts are added to the medium with multiple purposes such as buffering agents and promoters for solvent production.11,24,29 On the other hand, yeast extract and urea were used to support high hydrogen production from C. acetobutylicum NCIMB13357 and C. beijerinckii Fanp3, respectively.30,31
Nitrogen Source (5g/l) |
Cell Density (OD600)1 |
Xylanase Activity (U/ml) 1 |
Production1 |
|
Butanol (g/l) |
Hydrogen (l/l) |
|||
No nitrogen source |
3.8 |
2.18 |
2.55 |
0.81 |
Organic sources |
||||
Yeast extract |
4.28 |
2.31 |
3.15 |
0.82 |
Peptone |
4.12 |
2.24 |
3.1 |
0.72 |
Beef Extract |
4.2 |
2.16 |
3.05 |
0.7 |
Inorganic salts |
||||
Ammonium chloride |
3.98 |
2.55 |
3.11 |
0.85 |
Ammonium sulfate |
4.13 |
2.69 |
3.32 |
0.9 |
Sodium nitrate |
3.82 |
2.23 |
2.61 |
0.81 |
Potassium nitrate |
3.87 |
2.21 |
2.65 |
0.82 |
Urea |
4.01 |
2.38 |
3.09 |
0.87 |
Table 1 Effect of nitrogen source on butanol and hydrogen production.
1Average values obtained from three independent experiments
Optimization of culture medium by RSM
Based on conventional one-at-a-time optimization strategy, the preferable nitrogen source (ammonium sulfate) was selected to support simultaneous production of butanol and hydrogen in ABE fermentation. To understand interactive effects and also to attain suitable combination of carbon and nitrogen sources (C/N ratio) to maximise butanol and hydrogen production, RSM was employed. According to experimental strategy, the concentrations of xylan and ammonium sulfate were varied. The actual values and the corresponding coded levels for each independent variable are given in Table 2. Based on preliminary results, values corresponding to centre point on butanol and hydrogen production were chosen (data not shown). All the trials according to experimental plan were performed, and xylanase activity, butanol concentration and hydrogen concentration are given in Table 2.
Run No |
Variables and coded values |
Xylanase activity (U/ml) |
Responses |
||||||
Xylan (g/l) [X1] |
Ammonium Sulfate (g/l) [X1] |
Butanol (g/l) |
Hydrogen (l/l) |
||||||
Predicteda |
Actual |
Residual (%)b |
Predicteda |
Actual |
Residual (%)b |
||||
1 |
50 |
10 |
7.59 |
10.78 |
10.82 |
>0.3 |
3.11 |
3.12 |
>0.3 |
2 |
30 |
10 |
2.75 |
3.39 |
3.5 |
>3 |
1.01 |
0.97 |
<3.9 |
3 |
50 |
10 |
7.57 |
10.78 |
10.84 |
>0.5 |
3.11 |
3.14 |
>0.5 |
4 |
60 |
12.5 |
7.26 |
12.47 |
12.52 |
>0.4 |
3.38 |
3.44 |
>1.5 |
5 |
50 |
15 |
3.85 |
6.42 |
6.02 |
<6 |
2.3 |
2.29 |
<0.5 |
6 |
60 |
7.5 |
5.83 |
8.31 |
8.4 |
>1 |
1.79 |
1.86 |
>3.5 |
7 |
50 |
10 |
7.59 |
10.78 |
10.84 |
>0.5 |
3.11 |
3.14 |
>0.5 |
8 |
50 |
5 |
2.97 |
3.21 |
3.14 |
<2 |
1.25 |
1.26 |
>0.5 |
9 |
70 |
10 |
4.41 |
10.78 |
10.58 |
<2 |
1.8 |
1.82 |
>0.5 |
10 |
40 |
7.5 |
4.95 |
7.19 |
7.28 |
>1 |
2.15 |
2.1 |
<2 |
11 |
40 |
12.5 |
4.91 |
7.49 |
7.42 |
<1 |
2.19 |
2.18 |
<0.5 |
12 |
50 |
10 |
7.51 |
10.78 |
10.83 |
>0.5 |
3.11 |
3.12 |
>0.5 |
13 |
50 |
10 |
7.55 |
10.78 |
10.86 |
>0.5 |
3.11 |
3.1 |
<0.5 |
Table 2 Experimental plan and production of butanol and hydrogen.
Regression equations describing the relationship between the production of butanol and hydrogen and test variables in coded units are,
(2)
(3)
Where, X1 and X2 represents the concentration of xylan (g/l) and ammonium sulfate (g/l)
The coefficient of determination (R2) was calculated as 0.9875 and 0.9906 for butanol and hydrogen production (Table 3 & 4), indicating that the statistical model can explain 98.75 and 99.06% of variability in the responses respectively.32 Adequate precision measures signal-to-noise ratio. These values are found significant for butanol (29.65) and hydrogen (33.43) production. The predicted R2 values of butanol (0.9014) and hydrogen (0.9075) are in reasonable agreement with their adjusted R2 values of 0.9786 and 0.9839, respectively. They indicate good agreement between experimental and predicted values for butanol and hydrogen production. Noticeably, the model F-values of butanol (110.64) and hydrogen (147.97) and their values of P (<0.0001) indicate that model terms are significant. The lack of fit F-values of butanol (2078.59) and hydrogen (85.86) implies that the lack of fit is significant for both cases. Moreover, the coefficient of variations for butanol and hydrogen production are estimated 4.85 and 4.02% respectively, these low values indicate a better precision and reliability of the experiments.
Source |
SS |
DF |
MS |
F-Value |
Probability (P) |
Model |
108.49 |
5 |
21.7 |
110.64 |
<0.0001 |
Residual (Error) |
1.37 |
7 |
0.2 |
||
Lack of Fit |
1.37 |
3 |
0.46 |
2078.59 |
<0.0001 |
Pure Error |
8.80E-04 |
4 |
2.20E-04 |
||
Total |
109.86 |
12 |
Table 3 Result of ANOVA quadratic model for butanol production.
R2=0.9875; adjusted R2=0.9786; predicted R2=0.9014; adequate precision=29.657; coefficient of variation (C.V)=4.85%; SS - sum of squares, DF -degree of freedom, MS - mean square.
Source |
SS |
DF |
MS |
F-Value |
Probability (P) |
Model |
7.74 |
5 |
1.55 |
147.97 |
<0.0001 |
Residual (Error) |
0.073 |
7 |
0.01 |
||
Lack of Fit |
0.072 |
3 |
0.024 |
85.86 |
0.0004 |
Pure Error |
1.12E-03 |
4 |
2.80E-04 |
||
Total |
7.81 |
12 |
Table 4 Result of ANOVA quadratic model for hydrogen production
R2=0.9906; adjusted R2=0.9839; predicted R2=0.9075; adequate precision=33.430; coefficient of variation (C.V)= 4.02%; SS -sum of squares, DF - Degree of Freedom, MS: Mean Square
Student t-test and P-values of coefficients of linear, interactive and quadratic terms are listed in (Table 5). The model terms express linear(X1 and X2), interactive (X1X2) and quadratic (X12 and X22) effects of medium components on butanol and hydrogen. Based on t-value, the probability of the difference (P-value) in the data due to sampling error is calculated. If P- value measures lower than 0.05, the model term is significant and influences the response directly Myers et al.12 Accordingly, in the case of butanol production, the linear term of xylan (X1) measures low P-value (<0.0001), this indicates that xylan concentration in the medium majorly influences butanol production than ammonium sulfate (P=0.0003), whereas in hydrogen production ammonium sulphate concentration in the medium majorly impacts the hydrogen released than xylan (P=0.0001). Meanwhile, the quadratic terms of xylan ( ) as well as ammonium sulphate ( ) estimate much lower P–values (<0.0001) for both butanol and hydrogen production. Notably, the interactive terms (X1X2) of butanol (P=0.0028) and hydrogen (P=0.0002) productions are also found significant to a larger extent, which ensures that the synergistic effect of xylan and ammonium sulfate on butanol and hydrogen production exists. The interactive effect of these components on butanol and hydrogen production were further analysed by generating response surface plots (Figure 1).
Model Term |
Parameter Estimate |
Standard Error |
Computed t-Value |
P-Value |
||||
Butanol |
Hydrogen |
Butanol |
Hydrogen |
Butanol |
Hydrogen |
Butanol |
Hydrogen |
|
Intercept |
11 |
3.13 |
0.18 |
0.042 |
61.11 |
74.52 |
< 0.0001 |
< 0.0001 |
X1 |
1.7 |
0.23 |
0.13 |
0.03 |
13.08 |
7.67 |
< 0.0001 |
0.0001 |
X2 |
0.83 |
0.31 |
0.13 |
0.03 |
6.38 |
10.33 |
0.0003 |
<0.0001 |
X1X2 |
0.99 |
0.38 |
0.22 |
0.051 |
4.5 |
7.45 |
0.0028 |
0.0002 |
X12 |
-0.94 |
-0.43 |
0.093 |
0.021 |
-10.11 |
-20.48 |
< 0.0001 |
< 0.0001 |
X22 |
-1.55 |
-0.34 |
0.093 |
0.021 |
-16.67 |
-16.19 |
< 0.0001 |
< 0.0001 |
Table 5 Significance of regression coefficients.
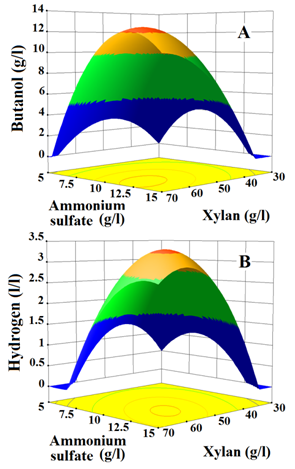
It shows that fermentation of fixed concentration of one component along with the increasing concentration of other component does not significantly enhance the production of butanol and hydrogen. Conversely, the concomitant increase in xylan (35 to 62.5g/l) and ammonium sulfate (6 to 12.5g/l) concentrations in the medium results in a steep increase in butanol and hydrogen production. Later, further increase in xylan (>62.5g/l) and ammonium sulfate (>12.5 g/l) concentrations in medium shows a sharp decrease in the production of butanol and hydrogen.
The levels of variables for maximum production of butanol and hydrogen estimated with high desirability factor (0.9570) from the models by numerical method according to Meyers and Montgomery, they are 58.4g/l for xylan and 11.8g/l for ammonium sulfate. These values fall within the range of experimental design.32,33 In an optimized culture medium (OCM) containing the components mentioned above, Clostridium strain BOH3 produced 12.15 g/l of butanol and 3.291 l/l of hydrogen, both values are close to predicted values 12.50g/l and 3.28l/l for butanol and hydrogen, respectively. Compared to butanol and hydrogen production in RCM, the Clostridium strain BOH3 produced 4-time higher butanol and hydrogen in OCM.
Butanol and hydrogen production in OCM
For BOH3, the optimal Carbon/Nitrogen (C/N) ratio for simultaneous production of butanol and hydrogen can be determined by using elemental analysis. For xylan, the carbon and nitrogen compositions are 40.66% and <0.5%, respectively. For RCM, the carbon and nitrogen composition are 33.29% and 6.52%, respectively. Meanwhile, ammonium sulfate contains 21.21% of nitrogen only. Based on these values, the total concentration of carbon and nitrogen served in optimized culture medium is estimated as 36.3 and 4.9g/l, respectively. Subsequently, the C/N ratio of optimized culture medium containing xylan (58.4g/l) and ammonium sulfate (11.8g/l) is calculated as 7.3. Furthermore, to evaluate the utilization of xylan, strain BOH3 was also cultured in RCM containing 58.4 g/l of xylose for comparison. In some cases, ammonium sulfate (11.8g/l) was also added to RCM to investigate the effect of ammonium sulfate on butanol and hydrogen production. Figure 2 depicts the fermentation profiles of strain BOH3 when it cultured in RCM with xylose only or xylose plus ammonium sulfate.
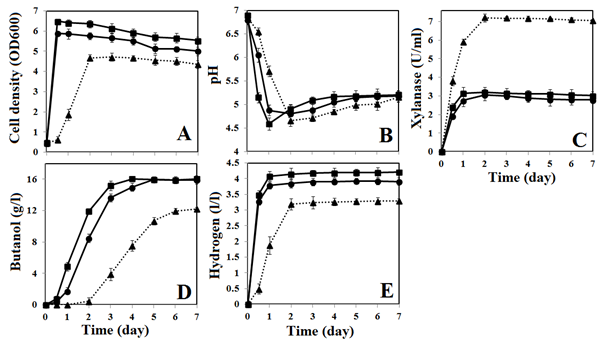
Obviously, xylose is the preferred carbon source for strain BOH3, hence the RCM containing xylose and xylose plus ammonium sulfate shows a higher cell density of 5.89 and 6.45, respectively. Both values are higher than the cell density of 4.63 from OCM. Meanwhile, RCM with xylose only or xylose plus ammonium sulfate leads to negligible lag time for strain BOH3, which attains its maximum cell density after 12h of fermentation. Conversely, there is a lag time of 12h in OCM for strain BOH3, which attains its maximum cell density after 48h of fermentation (Figure 2A). Because the pH in the culture medium was not controlled, when the strain BOH3 actively grows in RCM with xylose or xylose plus ammonium sulfate, it excretes acetic and butyric acids in the media and hence the pH drops to 4.88 and 4.59, respectively, within 24 h. In contrast, it takes 48 h before the pH drops down to 4.66 in OCM (Figure 2B). In terms of xylanase expression by strain BOH3, OCM supports the highest level of xylanase activity (7.15U/ml), followed by RCM with xylose plus ammonium sulfate (3.12U/ml) and then RCM with xylose only (2.76U/ml) (Figure 2C). From RCM with xylose and xylose plus ammonium sulfate media, the butanol production began at 12h and the maximum production (16.05±0.15g/l) is obtained after 5 and 4 days of fermentation, respectively. In OCM, butanol production started after 48h and reached maximum production (12.15±0.10g/l) after 7days of fermentation (Figure 2D). Similarly, in the case of hydrogen production, both RCM with xylose and xylose plus ammonium sulfate supported maximum hydrogen production after 24h of fermentation. The hydrogen concentrations can reach 3.81±0.12 and 4.15±0.15l/l, respectively. In contrast, BOH3 grown in OCM achieved its maximum hydrogen production (3.19±0.18 l/l) after 48h of fermentation (Figure 2E). Supplementing ammonium sulfate with xylose in RCM leads to a higher cell density than in medium contained only xylose (6.45 vs 5.89), which ultimately leads to rapid utilization of xylose and high butanol production from a relatively shorter fermentation time (4days). Additionally, RCM supplemented with xylose and ammonium sulfate shows slightly higher (~10%) hydrogen production than RCM with xylose only. While comparing OCM against xylose containing media, the butanol and hydrogen production is lower in OCM, probably because xylan in OCM is not fully utilized to support cell growth (cell density=4.68) and the production of butanol and hydrogen. Presumably, the amount of xylanase expressed (7.15±0.12 U/ml) in OCM was not sufficient to digest the high concentration of xylan (58.4g/l) in OCM. To further investigate this, a certain amount of commercial xylanase (0.5 to 3U/ml) was added to OCM at the beginning of fermentation and the cell density, butanol and hydrogen production were monitored. While increasing the initial concentration of xylanase from 0.5 to 2U/ml, there was a gradual increase in cell density, butanol and hydrogen concentrations. Addition of 2U/ml of xylanase caused a higher cell density at 5.51±0.09. Meanwhile, butanol and hydrogen concentration was increased to 15.74±0.15g/l and 3.89±0.08l/l, respectively. Notably, addition of xylanase (~2U/ml) at the beginning of fermentation also reduced the lag time to only 5h and the fermentation can be completed within 5days. Meanwhile, further increasing the xylanase concentration (>2U/ml) in OCM did not significantly enhance the cell density and the production of butanol and hydrogen. However, it shows that OCM supports high xylanase expression (7.15±0.12U/ml), the initial concentration of xylanase (~2U/ml) is found to be important for three important purposes:
In Clostridium fermentation, production of butanol and hydrogen are strongly influenced by a specific C/N ratio provided in the culture medium. For instance, C. acetobutylicum ATCC824 required the C/N ratio between 5 and 6.25 to support high butanol production when it was cultured in glucose and ammonium acetate/sulfate supplemented medium.24,29 Conversely, C. acetobutylicum P262 required a C/N ratio about 25.7 for high butanol production from the medium contained sagostarch and combination of nitrogen sources including yeast extract and ammonium nitrate.34 On the other hand, it was reported that C. acetobutylicum NCIMB13357 requires a C/N ratio of 70 to support high hydrogen production in a medium containing glucose and yeast extract.30 Overall, it can be concluded that suitable C/N ratios required for high butanol and hydrogen production from Clostridium fermentation is dependent on the strains and the composition of culture medium.15
Consolidated bioprocess for high butanol and hydrogen production
The OCM reported herein supports the production of 12.15±0.10 g/l of butanol and 3.29±0.15 l/l of hydrogen from 58.4 g/l of xylan. It also promotes the expression of xylanase by BOH3 (7.15±0.12 U/ml) in the culture medium. In addition to butanol and hydrogen, xylanase is also one of the valuable products obtained in this process. It can be easily purified with a yield of 38±2 % by a simple one-step anionic exchange chromatography using Q-sepharose column.27 Consequently, the purified xylanase (2-2.5U/ml) was added to the subsequent batch of BOH3 fermentation in OCM (Figure 3). Addition of xylanase (~2U/ml) at the beginning stage of fermentation significantly reduced the lag time (5-6h) and supported the maximum cell density (5.47±0.18) at 24h of fermentation. Eventually, in the xylanase supplemented consolidated bioprocess, maximum production of butanol (15.63±0.21 g/l) and hydrogen (3.81±0.05 l/l) can be achieved after 5days of fermentation and that is almost the same as the time required for maximum production of butanol and hydrogen from xylose supplemented medium. It was also observed that addition of xylanase (~2U/ml) did not reduce the expression of xylanase from OCM. In every cycle, the xylanase activity was close to 7.1U/ml. Interestingly, the developed consolidated bioprocess (CBP) shows the potential of utilizing high concentration of xylan (~60g/l) for butanol and hydrogen production.
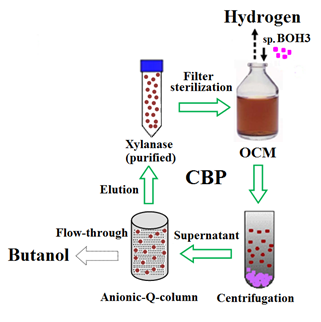
Overall, the developed CBP facilitates the possibility of recovering 28.14% of supplemented carbon as butanol and it also shows the potential of direct fermentation of xylan. This strategy can be used to eliminate the use of commercial xylanase and simple sugars such as xylose and glucose in the process. The concentrations of butanol and hydrogen produced by CBP are quite comparable to results observed in xylose-containing medium.
Optimized culture medium (OCM) which contains 58.4 g/l of xylan and 11.8 g/l of ammonium sulfate in RCM is suitable for culturing Clostridium strain BOH3 to produce butanol and hydrogen from xylan. In OCM, strain BOH3 can produce 12.15 g/l of butanol and 3.29 l/l of hydrogen (3.29l/l) and express xylanase (7.15U/ml) into culture medium. This xylanase can be purified and recycled in a consolidated bioprocess to further enhance the utilization of xylan. This consolidated bioprocess facilitates the possibility of recycling valuable xylanase and it assists in direct and effective fermentation of xylan to support high level of butanol and hydrogen production.
Clostridium strain BOH3 was gifted by Prof. Kun-Lin Yang, National University of Singapore (NUS), Singapore. All necessary facilities and support provided by Temasek laboratory (NUS), and South Asian University, New Delhi acknowledged.
The author declares no conflict of interest.

©2016 Rajagopalan. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.