Journal of
eISSN: 2572-8466


Review Article Volume 11 Issue 3
Department of Bioengineering, University of California, Los Angeles (UCLA), USA
Correspondence: Dr. Bill Tawil, Department of Bioengineering, UCLA School of Engineering, Los Angeles, CA 90095-1600, USA
Received: June 23, 2024 | Published: July 1, 2024
Citation: William K, Bill T. Diabetic nephropathy: Current treatments and tissue engineering strategies. J Appl Biotechnol Bioeng. 2024;11(3):64-71. DOI: 10.15406/jabb.2024.11.00362
Kidney complications are extremely common in diabetic patients, affecting around 40% of those with type 1 and type 2 diabetes, and is the leading cause of end-stage kidney disease. Due to increasingly processed foods and sedentary habits, diabetic nephropathy (DN) continues to grow in prevalence all over the world. In just the 21st century alone, the number of cases for chronic kidney disease nearly doubled. Kidney complications come in many forms such as hyperglycemia, glomerulosclerosis, proteinuria, and hypertension to name a few. Despite the severity of DN, a cure does not currently exist. At the later stages of diabetic nephropathy, dialysis and renal transplantation remain the only options and even the most advanced tissue engineering products have just entered early clinical trials. This paper provides an overview of the underlying causes of DN, a list of current treatments, and tissue engineering products in development.
Keywords: diabetic kidney disease, tissue engineering, chronic kidney disease, glomerulosclerosis
DN, diabetic nephropathy; ESRD, end-stage renal disease; eGFR, estimated glomerular filtration rate; TGF- β), transforming growth-factor beta; VEGF, vascular endothelial growth-factor; ROS, reactive oxygen species; CKD, chronic kidney disease; DALYs, disability adjusted life years; ARB, angiotensin receptor blocker; ACE angiotensin-converting enzyme; RAAS, renin-angiotensin-aldosterone system; SGLT2, sodium-glucose cotransporter-2; UCSF, University of California San Francisco; MSCs, mesenchymal stem cells; IL-6, interleukin 6; bFGF, basic fibroblast growth factor LIF, leukemia inhibitory factor; AWAK, automated wearable artificial kidney; AEs, adverse events
For both type 1 and type 2 mellitus, diabetic kidney diseases are major complications that when unchecked lead to kidney failure or end-stage renal disease (ESRD).1 Diabetic nephropathy is the name assigned to renal damage from diabetes, and is primarily caused by damaged blood vessels in the kidneys.2 Not only is it extremely common, appearing in around 30-40% of diabetic patients, but also is uncurable as there is currently not a single product that can completely reverse diabetic nephropathy.1 Hypertension - heightened blood pressure - is thought to be the main cause; however, there are many other possible causes such as insulin resistance, hyperglycemia, and an autoimmune response.1 In this paper, an overview of current efforts towards the treatment of diabetic nephropathy will be presented alongside a brief discussion of current market trends and diabetic population changes.3 Finally, products and novel strategies that are undergoing clinical trials will be summarized.4,5
Healthy tissue
Aside from being recognized as a central filtering unit within the body, kidneys are responsible for many other functions like maintaining the pH balance, blood pressure, and electrolyte levels as well as the secretion of important hormones (i.e. calcitriol, erythropoietin, and renin).6,7 As for their structure, kidneys are bean-shaped and can be divided into the cortex and medulla which are outlined in Figure 1a..7,8 Both of these regions are made up of nephrons, which waste products travel through from the renal corpuscle into the renal tubules and out the papilla/tips of the medullary pyramids where all of the urine empties into.6 Another essential subunit within the kidneys are the glomeruli, which are clusters of blood vessels that filter the blood and malfunctions of these vessels have been linked to the onset of diabetic nephropathy.9
Healthy kidneys are capable of filtering over 180 liters of fluid every day while reabsorbing the majority.6 A common test to determine how well the kidneys are filtering is through measuring the estimated glomerular filtration rate (eGFR).1 A high eGFR of around 90 mL/min is considered healthy while anything below may be an indication of early or developed kidney disease.1 The left kidney in Figure 1b is a generic representation of what a healthy kidney may look like, with its intact glomeruli and unobstructed tubules.9
Diseased tissue
Among the many causes for DN, hyperglycemia has one of the clearest connections to ESRD through the activation of several defined pathways.1 In addition to these pathways, significant renal inflammation can be caused by an increase of growth factors such as transforming growth-factor beta (TGF- β) and vascular endothelial growth-factor (VEGF).1 Vascular remodeling due to diabetes enhances blood pressure, or hypertension, which will ultimately give rise to detectable symptoms such as albuminuria.1,10
The production of reactive oxygen species (ROS) is a significant contributor to pathogenic pathways that can lead to DN and even cancer.11 Hyperglycemia due to diabetes is one such contributor to ROS production and disrupts glycolysis via the buildup of early glycolytic intermediates which initiate many damaging pathways.11 For example as shown in figure 1c, heightened glucose levels can lead to the polyol, hexosamine, PKC, and AGE pathways.11 Specifically, PKC has been shown to develop diabetes related complications such as nephropathy and neuropathy due to the disruption of vascular hemostasis by PKC isoforms.12 The onset of DN from hyperglycemia is caused by a myriad of other factors such as hypoxia, elevated triglyceride levels, excessive growth factor production, and endothelial dysfunction.11
Albuminuria, a hallmark symptom of DN, and the processes that lead to it have been a focal point for researching treatments.11 Glomerulosclerosis refers to damage to the renal glomeruli and results in proteinuria.13 A major contributor to glomerulosclerosis is thought to be disruptions to glomerular podocytes which, along with endothelial cells, work as a filtration barrier within the glomeruli of the kidneys (Figure 1d).11,14 Addressing hyperglycemia induced podocytopathy is one of the main avenues through which research has been directed for the reduction of albuminuria and restoration of normal glomeruli function.11
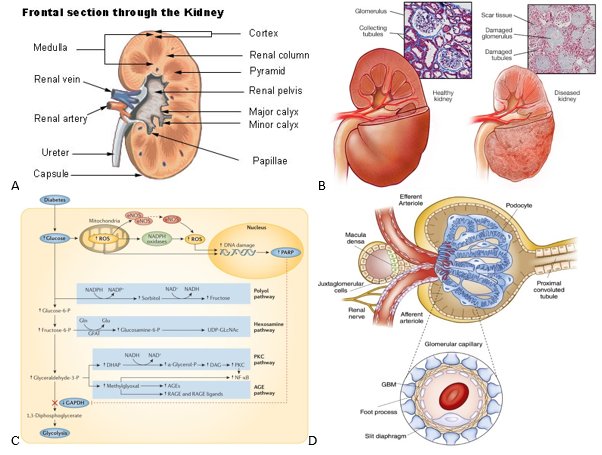
Figure 1 Diabetic nephropathy anatomy and pathways.
(a) Cross-sectional view of a healthy kidney is shown.8 (b) Histology slides and external view of healthy vs. damaged kidneys are shown.9 (c) Reactive oxygen species evolution is one of the main contributors to the damaging effects observed by diabetic nephropathy.11 (d) A cross-sectional view of the bowman’s capsule and its constituent glomeruli are displayed.14
Tissue damage from DN comes in several forms, most notably thickening of the glomerular basement membranes, glomerulosclerosis, and inflammation.1 Figure 1b shows the histology and changes to the structure of an effected kidney.9 As seen in the figure, a kidney affected by DN often has scar tissue formation alongside damaged glomeruli.9 Kimmelstiel-Wilson nodules are also hallmark attributes of diseased kidneys and are lesions that form in the glomeruli.1,15,16
The development of DN is divided into 5 stages.17 From stage 1 the patient experiences hypertrophy and renal hyperfunction, however the condition is somewhat reversible.17 Stage 2 is when the disease begins to develop silently with slowly elevating albumin levels until stage 3 where heightened albumin excretion becomes detectable. Stage 4 is where all the aforementioned symptoms grow significantly.17 Finally, end-stage renal disease or stage 5 is characterized by complete liver failure.17 Common symptoms of diabetic nephropathy include consistent albuminuria (>200 ug/min), worsening glomerular filtration rate (measures renal efficiency by estimating the volume of blood passing through the glomeruli per unit time), and heightened blood pressure.8,9
Type 2 diabetes is extremely prevalent, affecting one in every ten Americans, and about half of these patients develop a form of chronic kidney disease which overall affects a quarter of all diabetes patients.2,18 In America alone it can be estimated that around 20 million suffer from diabetic nephropathy or some sort of renal degradation due to diabetes.2 Additionally, type 2 patients tend to experience a higher chance of developing chronic kidney diseases (CKD) as they are generally older and CKD most commonly appear in people over the age of 45.2,11 The global market size for diabetic nephropathy was estimated to be 2.5 billion in 2022 and continues to rise every year at a compound annual growth rate of 5.3%.3 Furthermore, diabetic kidney disease patients account for a third of renal transplants and, of the 46,000 patients currently waiting for a kidney transplant, have the highest mortality rate.19 However, the amount of transplants performed every year has remained steady at around 24,000 to 26,000 (~12% reduction to waitlist size during COVID).20 The high mortality rate can largely be attributed to the high proportion of patients unaware of their significant kidney damage.18,21 Even at stage 4 of chronic kidney disease, the final stage before ESRD, only about 40% of individuals with CKD are aware of their own renal damage (Figure 2a).18 As patients progress into the later stages of CKD, dialysis and death rates rise significantly as seen in Figure 2b.20 Not only is kidney damage from diabetic nephropathy extremely prevalent, but it also incurs a significant economic burden to all patients.22 On average, healthcare costs range from $8,000 to $43,000 and continue to climb as one’s condition worsens.22
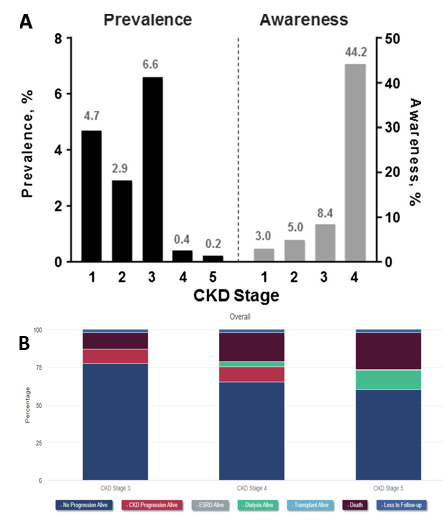
Figure 2 Burden of Chronic Kidney Disease (CKD) by stage and year in the United States.|
(A) Almost half of patients with CKD are not aware of any underlying kidney damage prior to stage 4.18
(B) Patient outcomes after one year of each stage are separated in the categories shown.20
There are several measures by which the spread of DN can be quantified over time such as DALYs.23 The disability adjusted life years (DALYs) is used as a measurement that sums the amount of years burdened and lost to the disease.23 DALYs due to chronic kidney disease from type 2 diabetes is shown below in Figure 3a.23 As evidenced by the graph, the burden that diabetic nephropathy places on the general public continues to increase with time even when adjusted for the increasing population size.23 Figure 3b displays the number of deaths in each country due to CKD, however, death rate is not directly related to number of cases as treatment efficacy varies significantly around the world.23 Each country is different in terms of susceptibility to DN due to factors like cultural differences, access to healthcare, and socioeconomic status.11 The market for treatments and drugs related to diabetic nephropathy continues to grow as the amount of cases of ESRD increases every year, nearly doubling after two decades (Figure 3c.).24 While not all conditions and cases for ESRD-related deaths are due to diabetic nephropathy, it is evident from these figures that renal diseases continue to quickly rise in prevalence.24
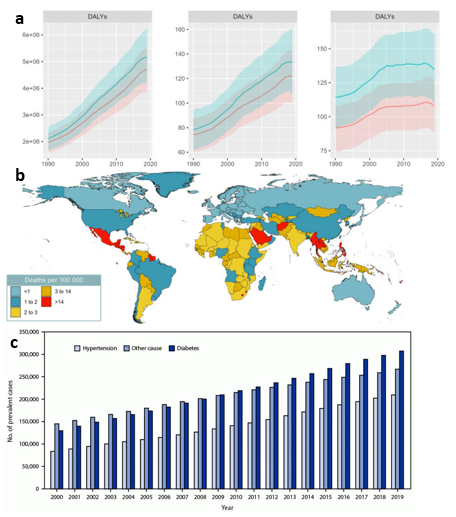
Figure 3 Global and national statistics for chronic kidney disease due to diabetes and ESRD.
(a) DALYs from 1990 to 2019 - with left to right being total number of years, crude rate (per 100,000), and age-standardized rate (per 100,000) – experienced continuous growth.23
(b) Death rate of chronic kidney disease due to type 2 diabetes is disproportionately distributed around the world.23
(c) The number of reported cases of ESRD in the U.S. has grown significantly in recent years.24
Due to transplant limitations, patients must turn to alternative treatments such as dialysis and medication to halt the progression of diabetic nephropathy.19,25
Hemodialysis
A dialysis machine acts as an external filter that pumps blood from the patient’s body and filters it by running the blood through the dialyzer.25,26 Inside, the dialysate a solution - made up of water, electrolytes, and salts – cleans the blood by removing waste products and reinserts the new filtered blood back into the patient.27 The market for dialysis machines is currently dominated by Fresenius, which accounts for the majority of dialysis machines used around the world.28 While most hemodialysis equipment is used in a clinical setting, there are several more compact devices that allow for dialysis at home (Figure 4).26 In addition, hemodialysis and peritoneal dialysis require a catheter to be inserted into the patient.29 The benefits and device descriptions are summarized below in Table 1.25,26

Figure 4 Selection of available in-center and in-home hemodialysis machines.
The figure shows two of the globally most prevalent hemodialysis machines.26
|
Device name |
Company |
Advantages |
Disadvantages |
Features |
|
2008T Bluestar |
Fresenius (Germany) |
- Highly customizable (wide choice of dialyzers, dialysate, and concentrate) |
- Bulky and takes up significant space (not as big of an issue inside the clinic) |
- Remote monitoring and data collection |
|
- Highly efficient |
- Requires strict scheduling for dialysis times |
- Touch screen makes for intuitive navigation |
||
|
- Requires patient to travel to facility (may be far away) |
||||
|
- Reduced privacy |
||||
|
NxStage VersiTM HD |
NxStage (US) |
- Extremely mobile (can provide therapy while travelling) |
- Away from medical professionals |
- Creates dialysate from just tap water |
|
- Does not require a large water purification system or complicated electrical setup |
- Requires electrical and plumbing setup, although not as complicated as other in-home devices |
- About the size of a normal end-table (around 70 lbs. with 15x15x15 in. dimensions) |
||
|
- In-home usage [alleviates transportation burden, higher flexibility of usage, comfortable use at home] |
||||
|
QDS: Quanta Dialysis System |
Quanta (UK) |
- Extremely compact (only at 520x370x550 mm) |
- Away from medical professionals |
- Disposable cartridge system allows for consecutive uses without disinfecting |
|
- In-home usage [advantages above] |
- Requires caretaker to deal with waste removal |
- Touch screen makes for intuitive navigation |
||
|
- Adjustable dialysate flow rate (from 500 mL/min to 300 mL/min) |
||||
|
- Saves time from disinfecting |
||||
|
Tablo |
Outset (US) |
- Easy and quick setup (around 20 minutes) |
- Away from medical professionals |
- Built-in wheels for transportation |
|
- In-home usage [advantages above] |
- Requires electrical and plumbing setup (simplified: only requires electrical outlet with tap water) |
- Touch screen makes for intuitive navigation |
||
|
|
|
- Alarm system for potential problems (constantly monitoring for kinks in tubing and irregular blood pressure) |
||
Peritoneal dialysis
Both peritoneal dialysis and hemodialysis are strategies employed in the later stages of diabetic nephropathy where kidney damage has progressed to an irreversible stage.11,25,30 Compared to dialysis machines, however, peritoneal dialysis devices present several advantages such as an often more compact design with lower costs, comparable survival rates, and completely internal filtering inside the body.31 As the name suggests, this treatment moves the dialysate across the peritoneal membrane while flooding any waste products.30,31 When dialysate floods the peritoneal cavity, there is an exchange between the solutes inside of the dialysate and the waste inside the bloodstream (urea, creatinine, and potassium).30,31 Similar to the dialysis machines, Baxter and Fresenius are prominent companies for peritoneal dialysis.32 Images of common peritoneal dialysis devices by their respective companies are shown below alongside a table of features for both (Figure 5) (Table 2).32
Figure 5 Selection of available peritoneal dialysis machines from Baxter (left) and Fresenius (right)
The figure shows two of the globally most prevalent peritoneal dialysis machines.32
|
Device name |
Company |
Advantages |
Disadvantages |
Features |
|
Amia |
Baxter (US) |
- Extremely portable and low-weight |
- Caretaker and patient must be concerned with waste disposal, device maintenance and usage, alongside setup |
- Voice-guided |
|
- In-home dialysis [has the same advantages listed above in the dialysis section] |
- No built-in system for minimizing contamination |
- Remote monitoring enabled |
||
|
- Easy-to-use |
||||
|
Liberty Select |
Fresenius (Germany) |
- Extremely portable and low-weight |
- Caretaker and patient must be concerned with waste disposal, device maintenance and usage, alongside setup |
- Touchscreen makes for intuitive navigation |
|
- Easy-to-use |
- When away from a facility and/or healthcare professionals, issues and troubleshooting may be difficult to handle |
- StaySafe feature minimizes contamination risks when connecting and disconnecting the device from the patient |
||
|
- Simple set-up |
||||
|
- Quick fill and drain times |
||||
|
|
|
- Custom drain options |
||
Table 2 Industry standard peritoneal dialysis devices32
Medication
While no single drug completely halts or cures diabetic nephropathy, there are several on the market and in development that substantially delay its progression.1,33 In several studies, angiotensin receptor blockers (ARBs) have been shown to be a successful strategy in minimizing albuminuria for type 2 diabetes.1,33ARBs, and similarly angiotensin-converting enzyme (ACE) inhibitors, take effect by suppressing the renin-angiotensin-aldosterone system (RAAS).34 Secreted by the kidneys, renin is an enzyme responsible for catalyzing the conversion of angiotensinogen to angiotensin I.34 ACEs in the body will then convert angiotensin I to angiotensin II which, when bound to its respective receptors, will induce many of the negative responses observed in kidney diseases such as heightened blood pressure, endothelial dysfunction, and albuminuria.33,34 Thus agonists that minimize angiotensin II complex formation is beneficial for diminishing these adverse effects and have been developed in the form of medication as shown below (Table 3).34
|
Drug type |
Medication |
Advantages |
Disadvantages |
Features |
|
Angiotensin receptor blockers (ARB) |
- Azilsartan |
- Can be used simultaneously with ACE inhibitors to have a synergistic effect |
- ARBs may induce hypertension or renal failure for those who are highly dependent on RAAS |
- ARBs are given to patients who are unable to tolerate ACE therapy |
|
- Candesartan |
- Well tolerated and side effects are minimal |
- Increased risk of hypotension, hyperkalemia, and renal malfunctions |
- 40 or 80 mg tablet |
|
|
- Irbesartan |
- Most effective only at the initial stages of diabetic nephropathy |
- Can lower blood pressure and renal degradation |
||
|
- Losartan |
||||
|
- Telmisartan |
||||
|
- Valsartan |
||||
|
Sodium-glucose cotransporter-2 (SGLT2) |
- Canagliflozin |
- Lowers risk of heart attack and stroke for type 2 diabetes patients |
- Recently FDA approved in 2013 with minimal studies on the effect of age on usage |
- Taken orally at 100 mg daily |
|
- Dapagliflozin |
- No sufficient studies for its impact on pregnancy |
- Reduces blood sugar levels |
||
|
- Empagliflozin |
- Can have adverse effects when mixed with other medication |
|||
|
Metformin |
- Fortamet |
- Positive effects for diabetic nephropathy include reduced albuminuria and protection of the podocytes |
- Many of the therapeutic mechanisms are still uncertain |
- Much like many of the other medications it is usually supplemented with changes in diet and exercise |
|
- Glucophage |
||||
|
- Glumetza |
||||
|
|
- Riomet |
|
|
|
In addition to ARBs, hypoglycemic medication such as sodium-glucose cotransporter-2 (SGLT2) inhibitors have been used to reduce glucose absorption.35 Similar to the aforementioned drugs, SGLT2 prevents harmful pathways with a particular focus on minimizing glucose levels in the blood (increased urinary glucose excretion).35
Finally, much like insulin injections metformin adjusts the body’s response to insulin.36 Blood sugar secretion levels are reduced to prevent diabetes as well as to treat the early stages of diabetic nephropathy.36 However, many of the mechanisms by which metformin has salutary effects on renal function remains largely uncertain.36 The features of these drugs are listed below in Table 3.
Tissue engineering products
Tissue engineering solutions for renal degradation remains a challenge as the kidneys are highly complex with over 26 cell types.4 Therefore, as of now most tissue-engineering strategies for kidneys are in development or at early clinical trials.4 As diabetic nephropathy progresses into the later stages, treatment becomes increasingly limited and eventually leaves dialysis and/or kidney transplantation as the only remaining options.4 Research groups, however, have recently focused efforts to developing an extracorporeal or even an implantable bioartificial kidney.41 While clinical trials have still not yet started, the University of California San Francisco (UCSF) has been developing an implantable artificial kidney comprised of two components: blood filter for processing incoming blood and a bioreactor lined with kidney cells for channeling the filtrate into the urine.41 This device, however, runs into common issues with implantable devices like difficulties with miniaturization and unintended immune responses.41 However, many other products are in development and other tissue engineering strategies will be shown throughout the remainder of this paper.
Tissue engineering has shown promising results in treating diabetic nephropathy through stem cell implantation, decellularization, and bioartificial kidneys.42
Stem cells
Implementation of stem cells into synthetic and natural polymer scaffolds have been a popular approach for tissue engineering strategies due to their high differentiation potential and self-renewal properties.42 Out of all the stem cell types, mesenchymal stem cells (MSCs) have shown the greatest promise for clinical trials.43 The amelioration of renal damage via intravenous injection of MSCs is caused by their secretion of vital cytokines like vascular endothelial growth factor (VEGF), interleukin 6 (IL-6), basic fibroblast growth factor (bFGF), and leukemia inhibitory factor (LIF) to name a few.43 These cytokines promote many beneficial pathways that prevent inflammation and promote angiogenesis.44 Despite the prevalence of MSCs, clinical trials for treating kidney damage is severely limited and have only a few ongoing studies with most still in just phase 1 and 2.5 The experiment NCT01843387, identified by its clinicaltrials.gov ID, is a MSC study that was completed in 2016 which showed the safety and efficacy of an IV infused with MSCs.45 No adverse effects were observed in patients who received the MSC treatment and after 12 weeks estimated glomerular filtration rate (eGFR) showed signs of improvement (Figure 6a).45 Other ongoing studies such as NCT02585622 and NCT04125329 are still in the early trial stages and observe the effects of bone marrow-derived mesenchymal stromal cells and umbilical cord mesenchymal stem cells respectively for diabetic kidney disease.5,46,47 Both studies measure changes to eGFR and albuminuria levels before and after treatment.46,47
Xenotransplantation
In addition to stem cell therapies, xenotransplantation has long been devised as a strategy for treating kidney failure.48 While xenotransplantation was held back by immune rejection concerns, gene editing advances have allowed for novel treatments.48 In 2022, the first clinical-grade porcine kidney xenotransplant was performed on a brain dead patient and made numerous advances (Figure 6b & 6c).49 Most importantly, the researchers were able to avoid hyperacute rejection through knockout of specific genes encoding carbohydrate xenoantigen production and observed the porcine kidney could withstand the higher blood pressure present in humans.49 Despite the promising finds of this study, only a few human trials have been conducted recently for xenotransplantation and almost none for decellularized organs due to underlying ethical concerns as well as a lack of significant substantiated evidence for effective xenotransplantation strategies.48
Bio-artificial kidneys
Aside from the implantable kidney designed by UCSF, there are many bio-artificial kidneys in development but many have not yet reached human testing stages.48 Regardless, some devices have made it to clinical trials such as the automated wearable artificial kidney (AWAK).50 As shown in Figure 6d, the device works by first removing fluid from the peritoneal cavity of the patient.50 The fluid-dialysate mixture then passes through the adsorber system where sorbents remove toxins while the dialysate is regenerated.50 The dialysate is then drained at the end of therapy which can be up to 7 hours.50 Ultimately, 15 patients were chosen to undergo clinical trials during which serum urea, creatinine, phosphate, and serum β2-microglobulin levels were monitored.50 With AWAK therapy, levels of all these waste products were reduced; however, patients began experiencing adverse events (AEs) after about one month of therapy, most commonly abdominal pain/discomfort.50 Regardless, both the AWAK and implantable bioartificial kidney designed by UCSF are recent advances to an otherwise relatively stagnant field.50
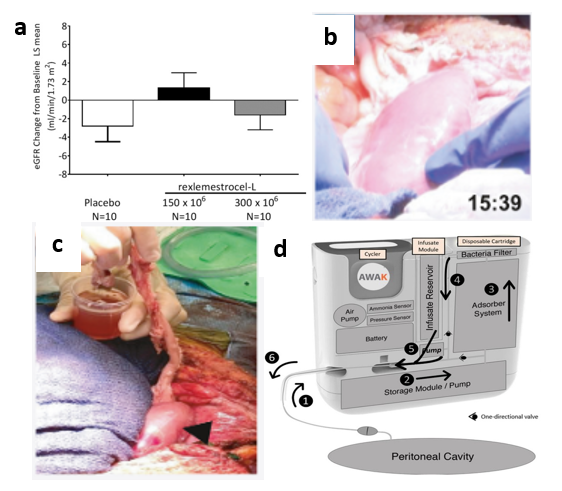
Figure 6 Ongoing tissue engineering research for diabetic nephropathy.
(a) An enhanced EGFR was observed with MSC intravenous MSC implantation into human subjects.45
(b) Transplantation and blood perfusion of porcine kidney in human patient was performed for the first time in 2022.49
(c) After perfusion, the right kidney produced around 700 cc of urine after 24 hours while the left kidney hardly produced any.49
(d) First automated wearable artificial kidney used for human trials is shown.50
Diabetic kidney disease, or diabetic nephropathy, is the leading cause of end stage renal failure and has attracted significant research interest towards halting its progression and reversing damage.1 Affecting around 20 million Americans, diabetic nephropathy continues to grow in prevalence with a large estimated market size of $2.5 billion with a 5.3% annual growth rate.3 In the early stages of its development, diabetic kidney disease and its symptoms (elevated blood pressure, elevated blood-glucose levels, etc.) are usually treated with medication, most notably angiotensin receptor blockers and sodium-glucose cotransporter-2.1 However, aside from albuminuria and lower eGFR levels, diabetic kidney disease often goes undetected allowing it to develop into its later stages.18As a result, hemodialysis, peritoneal dialysis, and kidney transplants remain the only options for treatment.11 Tissue engineering approaches in the form of mesenchymal stem cells, xenotransplantation, and bio-artificial kidneys have been employed to meet the current limitations in treatment available for late-stage diabetic kidney disease.11 However, tissue engineering for diabetic nephropathy is held back by hurdles in current technology to mimic the complex microstructures found in kidneys as well as the limited record of tissue engineering clinical trials.4,5 Regardless, novel strategies continue to push the envelope and may one day alleviate the burden of millions(Appendix).41,50
The authors acknowledge the Henry Samueli School of Engineering & Applied Science and the Department of Bioengineering at the University of California, Los Angeles. William Kwak also expresses specific appreciation to the guidance and knowledge provided by Professor Bill Tawil which greatly contributed to this paper’s production.
Authors declare that there is no conflict of interest.
None.

©2024 William, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.