Journal of
eISSN: 2572-8466


Research Article Volume 1 Issue 3
Department of Applied Sciences, UCSI University, Malaysia
Correspondence: Hooi Ling Ho, Faculty of Applied Sciences, UCSI University, No. 1, Jalan Menara Gading, UCSI Heights, Cheras, 56000 Kuala Lumpur, Malaysia, Tel 6012-3699854
Received: September 29, 2016 | Published: December 30, 2016
Citation: Ling HH. Batch submerged fermentation in shake flask culture and bioreactor: influence of different agricultural residuals as the substrate on the optimization of xylanase production by Bacillus subtilis and Aspergillus brasiliensis . J Appl Biotechnol Bioeng. 2016;1(3):96-104. DOI: 10.15406/jabb.2016.01.00016
Enzymes including xylanase produced by microorganism sources are preferable in many industries especially in baking, food, pulp and paper. Nonetheless, the costs of enzymes production in industry are considerably astronomical. Therefore, agricultural residuals such as barley husk and wheat bran have been introduced as alternate carbon source to reduce the production cost. The aim of this study is to elucidate the enzyme activity of xylanase by Bacillus subtilis and Aspergillus brasiliensis in shake flask culture and bioreactor using barley husk and wheat bran as carbon source under batch submerged fermentation, respectively. Based on the results obtained, using barley husk as the substrate in the fermentation process, the maximum xylanase activity of 3.305±0.143U/ml by B. subtilis was detected in shake flask culture. Surprisingly, much higher activity of 13.069±0.193U/ml was achieved in bioreactor. On the other hand, xylanase activity by A. brasiliensis was found to be 2.175±0.103U/ml after using wheat bran as the prime carbon source in flask culture. Indeed, much higher production of 7.074±0.089U/ml was observed in batch bioreactor system. Simple and defined carbon sources including glucose and pure substrate such as xylan, for example generally pricey that would upsurge the enzyme production cost. Thus, in order to produce cost-conscious xylanase, agricultural residuals are introduced into the batch submerged fermentation process. Some agricultural residuals that are heaving with sugars and nutrients are anticipated to effectively utilize by microorganisms besides being ease accessible and cost-effective. Based on the results in this study, barley husk and wheat bran are suggested to be affordable alternate carbon source for xylanase production in industries.
Keywords: xylanase, wheat bran, barley husk, bacillus subtilis, aspergillus brasilienisis, batch fermentation
BSA, bovine serum albumin; PDA, potato dextrose agar; ATCC, american type culture collection
Xylanase is an enzyme that breakdown xylan into xylose. It is produced mainly by microorganisms and used in the breakdown of the component of plant cell walls.1 Xylanase began to be useful in the 1980s in animal feed production and is now being used in various industries. Xylanase is the key enzyme in paper industry as bleaching agent, employed for clarifying juices and wines, extracting coffee, improving nutritional properties of agricultural silage and grain feed. Nevertheless, producing commercial xylanase is rather expensive and thus, production cost is a major concern for many industries. Researchers are still undergoing these days to find the best way to increase the yield and yet reducing the production cost. To lower the cost, agricultural residuals have been used as the carbon sources to produce microbial enzymes.2 Studies from Sugumaran KR, et al.3 proved that agricultural wastes such as rice husk, cassava bagasse and sawdust could be utilized as the carbon source for the production of microbial enzymes. Other examples of agricultural residuals such as barley husk and wheat bran are also anticipated as the substrate for enzymes production including xylanase. Barley husk is mainly composed of lignocellulosic cell wall with high amounts of polysaccharides including 39% cellulose, 22% lignin, 12% hemicelluloses, 11% starch and small amount of fibers, proteins, resins and antioxidants.4 The important sugars content found in barley husk made it suitable to be used as the carbon source for fermentation process. Besides being a cheaper substrate, barley husk is also used due to its relatively small particle size that possesses larger surface area for microbes to utilize. However, much smaller particle size of residuals would cause agumulation that interfere the microbial respiration where heat and carbon dioxide transfer would not be efficient in a pack situation.5 On the other hand, wheat bran is also another agricultural residual used as alternate carbon source for enzymes production. Wheat bran that is a by-product of flour milling, contains approximately 17% protein and significant 70% carbohydrates of which about 80% is consisted of cellulose and hemicelluloses.6 Consequently, it is considered as a good carbon source due to its remarkable nutritional content, large surface area and hence, it is suitable for xylanase production. In addition, wheat brain, besides containing xylan, its protein content is also suitable to use as nitrogen source.7 In fact, wheat bran also shows its greater prospect economically and competently in submerged fermentation because of its better air circulation, cheaper cost, looser particle binding and efficient penetration of mycelia.8 As a result, wheat bran shows greater significant enzymes activity and production for fungi as compared to other substrates.9 All in all, agricultural residuals, besides being able to reduce the costs of enzymes production but also manage to prevent pollutions to the environment. Since the demands of xylanase is getting grander, the involvement and utilization of agricultural residuals in both shake flask culture and bioreactor in this study would be of a good reference for the production of this enzyme in industries. Therefore, the objective of this study is to elucidate the enzyme activity of xylanase produced by B. subtilis and A. brasiliensis using barley husk and wheat bran as the substitute carbon source in shake flask and bioreactor in batch submerged fermentation respectively.
Microorganisms of Bacillus subtilis ATCC 6633 and Aspergillus brasiliensis ATCC 16404
Bacillus subtilis ATCC 6633 and Aspergillus brasiliensis ATCC 16404 were obtained from American Type Culture Collection (ATCC), Manassas, USA.Subsequently, a sterile inoculation loop was used to subculture the bacteria onto nutrient agar. Nutrient agar is a general bacterial growth agar that is suitable to subculture B. subtilis. B. subtilis is a non-fastidious microorganism which does not require complex nutrients to grow. After that, the culture agar plates were sealed with para-film and incubated at 37°C for two days before used as inoculum for shake flask culture and bioreactor. On the other hand, A. brasiliensis was subcultured on potato dextrose agar (PDA). PDA was used as it was relatively rich agar for growing fungi and most fungi thrive on PDA. Subsequently the culture agar plates were sealed with para film and incubated at 30°C for a week before used as inoculum for shake flask culture and bioreactor in this study.
Pre-treatment of barley husk and wheat bran
Barley husk and wheat bran were flaky prior to pre-treatment. Hence, barley husk and wheat bran were homogenized into finer and smoother particles using stainless steel blender before subjected to sieving to obtain their consistency in sizes. This process was carried out to ensure that B. subtilis and A. brasiliensis possessed better accessibility to these carbon sources of the agricultural residuals, respectively. In fact, much constant sizes of barley husk and wheat bran were easier to disperse and dissolve in the liquid culture medium of the batch submerged fermentation used in this study. After achieving the desired sizes of the barley husk and wheat bran, they were dried in oven at 65°C until their constant weights were achieved before used in this study.
Preparation of standard inoculum, medium formulation and xylanase production by Bacillus subtilis in shake flask culture
B. subtilis was subcultured on nutrient agar and incubated at 37°C overnight. Then, culture flask with a working volume of 250ml medium was prepared as the following (gram per liter): pre-treated barley husk-20; peptone-5; yeast extract-5; KH2PO4-1 and MgSO4.7H2O-0.1 in a 500ml culture flask. Subsequently, the initial medium culture pH was adjusted to pH 6.5 and the carbon source of barley husk was separately autoclaved from other medium compositions at 121°C for 15min to prevent Millard reaction. After cooling down the culture flask was inoculated with a standard inoculum size of 1 × 106 cells for xylanase production. Samples of batch fermentation were taken every 12h for analysis.
Preparation of standard inoculum, medium formulation and xylanase production by Bacillus subtilis in bioreactor
To perform the scale up of xylanase production in a bioreactor, a standard inoculum size of 106 cells of was inoculated into a flask culture with 250ml working volume. When the bacteria achieved the log phase, 100ml of the bacteria culture were inoculated into 1l medium culture in a bioreactor. The medium culture for the bioreactor was prepared as the following (gram per liter): pre-treated barley husk-20; peptone-5; yeast extract-5; KH2PO4-1; MgSO4.7H2O-0.1, MnSO4.4H20-0.5; CuSO4.5H20-0.5 and FeSO4.7H20-0.01 where the carbon source was prepared and autoclaved separately from other medium compositions. The initial medium pH was adjusted to pH 6.5 before autoclaved. Medium pH was then regulated at pH 6.5 during the batch fermentation process in the bioreactor. Samples of fermentation were taken every 12h for analysis.
Preparation of standard inoculum, medium formulation and xylanase production by Aspergillus brasiliensis in shake flask culture
In this study, A. brasiliensis was subcultured on PDA for a week. A culture flask with working volume of 250ml was prepared in a 500ml culture flask according to the formulation as stated (gram per liter): pre-treated wheat bran-20; yeast extract-4; KH2PO4-1.52; MgSO4.7H2O-0.52 and KCl-0.52. The initial pH medium was adjusted to pH 6.5 before the carbon source was separately autoclaved from other medium compositions at 121°C for 15min. Subsequently, the culture flask was inoculated with a standard inoculum size of 106 spores for xylanase production in batch submerged fermentation. Samples of fermentation were taken every 24h for analysis.
Preparation of standard inoculum, medium formulation and xylanase production by Aspergillus brasiliensis in bioreactor
To perform the scale up of xylanase production in a bioreactor, a standard inoculum size of 106spores of A. brasiliensis was inoculated into a flask culture with 250ml working volume. When the fungi attained log phase, 100ml of the fungal culture were inoculated into 1l medium culture of the bioreactor. The medium culture for the bioreactor was comprised of the following (gram per liter): pre-treated wheat bran-20; yeast extract-4; KH2PO4-1.52; MgSO4.7H2O-0.52; KCl-0.52; MnSO4.4H20-0.5; CuSO4.5H20-0.5 and FeSO4.7H20-0.01. The initial medium pH was adjusted to pH 6.5 before the carbon source was separately autoclaved from other medium compositions at 121°C for 15min. pH of the medium in the bioreactor was then controlled at constant pH of 6.5. Samples of fermentation were withdrawn every 24h for analysis.
Sampling and analysis
In this study, the samples of batch fermentation were used for the quantification of biomass concentration and measurement of medium pH. Subsequently, the samples were centrifuged at 10,000 rpm for 15min to obtain supernatant that used to determine xylanase activity and protein assays. All of the experiments were performed triplicate and mean values of the results were generated from analysis.
Xylanase activity
Xylanase activity was measured according to Bailey MJ, et al.10 To prepare the substrate for the xylanase activity, 1% of beech wood xylan was dissolved into 0.05M sodium phosphate buffer, pH 5.3 at 50°C. The xylan mixture was then mixed with 0.1ml supernatant and incubated at 50°C for 30min. The DNS method was then carried out and absorbance readings were measured at 575nm. The activity of xylanase was measured according to xylose standard curve. Xylose standard curve was plotted based on the absorbance reading at 575nm against its concentration. One unit of xylanase activity is defined as the amount of enzyme required to release one µmole of xylose per ml of enzyme extract per min under assay condition.
Soluble protein assay
The total amount of soluble protein in supernatant was determined according to Lowry method using Bovine Serum Albumin (BSA) as the protein standard.11 The absorbance reading of protein assay was measured using a spectrophotometer at 750nm. The protein assay was analyzed based on the BSA standard curve of absorbance reading at 750nm against its concentration.
Quantitative of biomass concentration
In this study, cells of B. subtilis and spores of A. brasiliensis were measured using haemocytometer.
Measurement of medium pH
The pH of the samples was measured using pH meter to elucidate the pH profile of B. subtilis and A. brasiliensis in batch submerged fermentation respectively. The pH meter was calibrated using buffer pH 4, 7 and 10.
Agricultural residuals as the carbon source for production of xylanase in batch submerged fermentation: Overview
Xylanases are hydrolytic enzymes that cleave randomly at the β-1,4 backbone of the complex plant cell wall polysaccharide of xylan.12 Xylanases are grouped into two families, F and G based on hydrophobic cluster analysis and sequence homology.13 Families F and G correspond to Families 10 and 11 in the numerical classification of glycosyl hydrolase. Family 10 endoxylanases is described as a 8-fold β/α-barrel while Family 11 endoxylanases form a simple single ellipsoidal domain comprising two β-sheets and a single three-turn α-helix.14 Several microorganisms including bacteria and fungi have been reported to be readily hydrolyzing xylans by synthesizing 1,4-β-D endoxylanases (E.C. 3.2.1.8) and β-xylosidases (EC. 3.2.1.37).15 Notably, xylanolytic enzymes from microorganisms have attracted a great deal of attention in the last decade, particularly because of their biotechnological characteristics in various industrial processes.16 As a result, xylanase was elucidated in this study because it constitutes of major commercial proportion of hemi cellulases that possess potentials in many industrial applications.12 In fact, xylanase gained much attentiveness in biotechnology due to their applications in the manufacturing industries such as paper and pulp, animal feed, food and fermentation.17 Besides that xylanolytic enzymes are being used for clarifying juices and wines, extracting coffee, plant oils and starches improving nutritional properties of grain feed and agricultural silage.18 The future prospects of xylanase are bright especially in environmental science such as bio-fueling, agro-waste and effluent treatment.15 Agricultural residuals have been introduced as carbon source in the process of enzymes production as they are cheap in cost. In addition, optimizing the usage of agricultural residues from agriculture and food industry is able to help to reduce environmental pollutions.19 Besides that, agriculture wastes are rich in nutrients that could be used to support the growth of microorganisms, and in return, produced valuable enzymes including xylanase.20 According to Norazlina I, et al.21 they showed that agricultural residuals such as oil palm fronds were used as the carbon source to produce xylanase. In this study, barley husk and wheat bran had been selected as the carbon source to utilize by B. subtilis and A. brasiliensis, respectively. These agricultural residuals as the carbon source were also studied by Kavya V, et al.9 and Bakri Y, et al.22 which showed significant results on xylanase production by A. niger. Nonetheless,xylanase production by A. brasiliensis has not been studied extensively.
In this study, B. subtilis and A. brasiliensis produced xylanase in shake flask culture and bioreactor under batch submerged fermentation using agricultural residuals of barley husk and wheat bran as carbon source under their optimum growth conditions. First of all, the carbon source used for the fermentation for the optimum xylanase production was barley husk and wheat bran for B. subtilis and A. brasiliensis, respectively. They were selected mainly due to their hemi cellulose content similarly to xylan. Notably, the study by Dobrovici PE, et al.4 showed that barley husk contains 12% of hemicelluloses. Hassan GE, et al.6 indicated that wheat bran contains about 43% of hemicelluloses. From their reports, they showed that wheat bran is a good carbon source of xylanase production as it contains high amount of hemicelluloses. Besides that, Malathi S, et al.8 observed that the loose binding particles of wheat bran lead to the efficient binding of mycelia. Therefore, wheat bran was chosen as the optimum carbon source for A. brasiliensis in this study. On the other hand, barley husk was used instead of wheat bran for the fermentation of B. subtilis in xylanase production. Yamada H, et al.23 reported that hemicelluloses prepared from wheat bran produced acidic polysaccharides. As a result, this condition was suitable for A. brasiliensis that favored slight acidic condition at pH 6.5. On the other hand, the study of Ecaterina DP, et al.24 showed that barley husk possesses higher thermal stability. Therefore, barley husk was chosen as the optimum carbon source for B. subtilis that required higher growth temperature than A. brasiliensis. Besides carbon source, the optimum growth conditions are important for the maximum production of xylanase. Growth conditions such as incubation temperature, agitation speed and medium pH also affect the outcome of xylanase production in batch submerged fermentation.
Submerged fermentation utilizes free flowing liquid culture substrates that is best suited for microorganisms such as bacteria that require high moisture content.25 Majority of microbial industrial enzymes that are extracellular hydrolases including xylanases are usually produced by aerobic submerged fermentation.26 In submerged fermentation, microorganisms are grown in a fully liquid system that has the advantage of control over the process parameters such as temperature, medium pH, aeration and dispersion for efficient growth and yield of the infective units.27 A steady-state production succeeds when all the parameters are under control and hence optimizing the fermentation process.28 Most importantly, submerged fermentation is an easier enzymes production process for upcoming enzymes purification steps.25 In fact, major industrial enzymes including xylanase are extracellular enzyme that would remain in the fermentation broth after biomass is removed and thus, resulting an easier purification process. As a result, submerged fermentation becomes the choices from enzymes production and purification.28 Therefore, xylanase production in this study was investigated using batch submerged fermentation approach followed by elucidation of its specific enzyme activity to determine its potential in purification as part of the downstream processing procedure, which could be used as a reference for the study of xylanase production in many industries.
Xylanase production by Bacillus subtilis in shake flask culture and bioreactor
In this study, agricultural waste of barley husk was used as the carbon source for the production of xylanase by B. subtilis under the optimum growth temperature at 37°C. Although bacteria may withstand different temperatures but the growth temperature, which is lower than the optimum, would inactivate the cells while higher temperature would in return, destroy the cells. Korsten L, et al.29 stated that the optimum temperature range for the growth of B. subtilis was occurred from 30°C to 37°C. In fact, optimum growth temperature of 37°C for B. subtilis was also used in the study by Budde I, et al.30. Similarly, Kuancha C, et al.31 discussed that the incubation temperature lower than 28°C and higher than 40°C were not suitable for xylanase production by B. subtilis. Production of xylanase was achieved at 37°C obtained from the study of Sepahy AA, et al.32 which yielded about 249.308 U/ml. Figure 1 shows the production of xylanase by B. subtilis in shake flask culture and bioreactor under the optimum growth temperature in this study. Based on Figure 1, the xylanase activity of B. subtilis reached its maximum activity at 48h in shake flask culture and bioreactor. Sanghi A, et al.33 stated that the xylanase production of 410U/ml was obtained under the optimized batch submerged fermentation conditions at 48h. Indeed, another study also agreed by Irfan M, et al.34. Their study showed that the maximum xylanase activity was gained at 48h with the production of 439.5U/ml. Furthermore, Gupta U, et al.35 also observed the maximum xylanase activity of 9.11U/ml at 48h in submerged fermentation. Based on our study, the maximum xylanase activity of 3.305± 0.143U/ml was obtained using barley husks in shake flask culture compared to 13.069± 0.193U/ml from bioreactor at 48 h of batch fermentation. It showed an increase of xylanase activity in scaling up approximately around 295% was achieved using bioreactor.
However, after 48h the production of xylanase was reduced in both shake flask culture and bioreactor, respectively. The reason that affected the decline of xylanase activity as reported by Sanghi A, et al.33 was due to the formation of thick suspension and improper mixing of substrates in the culture as the bacteria population size increased. When there was a large population size, the medium became a thick suspension. Therefore, it was hard for the substrates to mix well with the bacteria culture and hence, some of the cells in the population gained lesser nutrients. As a result, it gradually decreased the amount of cells and thus reduced the production of xylanase. Besides that, as the fermentation process proceeded, culture velocity and shake flask size would affect the hydrodynamics in the shake flask that indeed would reflect the growth of the cultured cells.36 Thus, larger 500ml shake flask was selected as the size used in the present study to promote better mixing and smoother agitation of the culture medium. In addition, when the incubation hours increased, Espinar MTF, et al.37 stated that smaller amount of enzymes such as lysozymes were released by the aging cells which entered the stage of autolysis. This might be another reason that supported the fact that xylanase activity decreased as fermentation prolonged. As for bioreactor, foam formation on the surface of the medium overtime would hinder the diffusion of oxygen into the medium and hence, lower the oxygen transfer rate to the bacteria cells. Nonetheless, this problem could be solved by breaking the foams using anti-foam agent or adjusting the level of the top propeller in bioreactor to the level same as the surface of the medium. On the other hand, the biomass production for this study is shown in Figure 2. At 48 h where the peak of xylanase activity obtained, the cell count also reached its maximum number, producing 1.54 ×109 ± 0.064 cells/ml in shake flask culture. Scaling up in a bioreactor showed an increment of 1033% in cell count which was 1.709×1010 ±0.023 cells/ml at 48h. Cell count under submerged fermentation in the shake flask culture and bioreactor showed steeper increase in gradient from 12h to 48h compared to other time as shown in Figure 2. This happen when the initial medium composition and optimum growth condition promoted the cell count that directly affected the xylanase activity. As indicated by Richana N, et al.38 when the efficiency of substrates consumption increased, the efficiency of enzymes production by cell biomass also increased. As the batch submerged fermentation proceeded, the cells reached the stationary phase in the shake flask culture, the nutrients including carbon source would gradually became inadequate to support the rapid cell reproduction and eventually started to deplete rapidly. Likewise, in a bioreactor, the initial larger amount of substrate was able to cope with the rapid cell reproduction rate and hence, upheld larger xylanase production. Besides that, extra manganese and copper in the medium composition that acted as enhancer and inducer for cell growth allowed the sharp increase of biomass and xylanase production simultaneously. Biomass production was greatly affected the xylanase production as shown in the correlation graph between the production of biomass and xylanase activity in Figure 3. When there was an increase in biomass production, the xylanase activity increased. Xylanase activity was directly proportional to the cell count of B. subtilis.
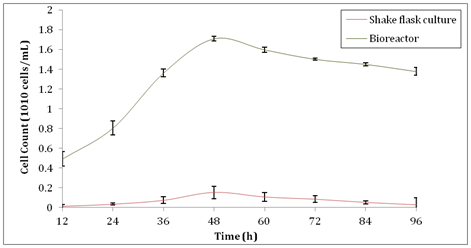
Figure 2 Cell count of B. subtilis during production of xylanase in shake flask culture and bioreactor.
Besides that, the xylanase activity was also affected by pH of the medium culture. Sepahy AA, et al.32 stated that pH of the medium strongly affected the enzymatic process. Figure 4 shows the trend of medium pH changed over fermentation time. Basically it increased over time as fermentation prolonged. Similar trend was also observed in the study of Kapoor M, et al.39 Consequently, pH became more alkaline over fermentation time due to the generation of basic amides.40 Notable, the study by Ouoba LI, et al.41 also corresponded to the issue of the increase of medium pH during fermentation process. They stated because of the degradation of free basic amino acids such as lysine released into the medium, the pH increased. Based on this study, the maximum xylanase activity and biomass production occurred at pH 7.52 at 48h under batch submerged fermentation. Similarly, according to Ayyachamy M, et al.42 they reported that the optimum and stability of xylanase occurred between pH 6 to pH 8. Besides that, many bacteria possessed the optimum pH for xylanase activity occurred from pH 7 to pH 9.43 When the pH of the medium changes, it changes the condition of the medium that may not favor the growth of cells and production of xylanase. When it becomes more alkaline, it causes base stress to the cells and thus, terminates the growth of the cells and eventually forms the endo-spores.40 As a result, it affects the xylanase production. There would be lesser cells to produce xylanase and hence, a reducing trend of xylanase activity as shown in Figure 1 & 2 after 48h. Furthermore, xylanase production is sensitive to medium pH.44 Medium pH that does not suitable to the enzyme activity would degrade the enzyme and cause the reduction in enzyme activity. Nevertheless, in a bioreactor, the medium pH was well monitored and controlled. This would provide an optimum pH condition for the cell growth and also the production of xylanase. As a result, there was higher xylanase activity produced using a bioreactor compared to shake flask culture in general.
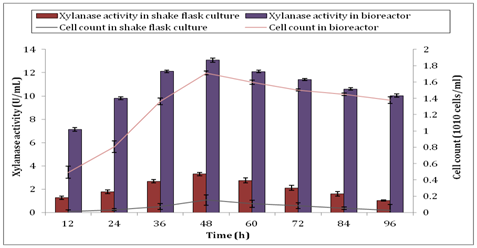
Figure 3 Correlation between xylanase activity and cell count by B. subtilis in shake flask culture and bioreactor.
On the other hand, in Figure 5, it shows the soluble protein production by B. subtilis in shake flask culture and bioreactor. The highest protein concentration produced by shake flask culture was 2.122±0.131g/ml. Notably, the production of protein in bioreactor was higher than shake flask culture with the maximum peak of 6.031±0.102g/ml at 48h. This result was used to calculate the specific enzyme activity of xylanase produced. Specific enzyme activity is calculated as enzyme activity/protein concentration. It is essential to determine how much protein was present during purification of enzyme as the purity is depended on the removal of unwanted proteins. Specific enzyme activity is a measurement for enzyme purity. The larger amount of specific enzyme activity the purer the enzyme. The specific enzyme activity for xylanase in this study at 48h was 1.557U/g for shake flask culture and 2.167U/g for bioreactor respectively.
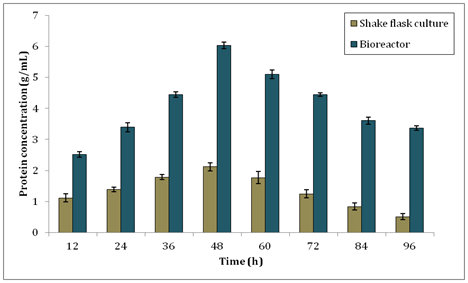
Figure 5 Protein concentration of B. subtilis during production of xylanase in shake flask culture and bioreactor.
Xylanase production by Aspergillus brasiliensis in shake flask culture and bioreactor
Wheat bran was used as the prime carbon source for the maximum production of xylanase by A. brasiliensis under the optimum growth condition in this study. Xylanase activity by A. brasiliensis in shake flask culture and bioreactor is shown in Figure 6. Based on the results obtained, the xylanase activity reached its maximum peak at 48h of batch fermentation, producing 2.175±0.103U/ml in shake flask culture. On the other hand, the maximum xylanase activity with 7.074±0.089U/ml was observed at 48 h in bioreactor. There was approximately around 225% increase in xylanase production using wheat bran as the carbon source in bioreactor. Instead of using xylan as the substrate, agricultural residuals were used as the carbon source for enzymes production including xylanase. Indeed, Huitron C, et al.2 showed that the xylanase activity of 1.52U/ml was produced by Bacillus sp. using Agave tequilana waste as carbon source at 48h. Furthermore, Tasneem M, et al.45 also measured the maximum xylanase activity of 215U/ml produced by Aspergillus sp. at 48h using wheat bran. Likewise, Guimaraes NCA, et al.46 reported that the maximum xylanase activity of 6.23U/ml was obtained using wheat bran in shake flask culture at 48h. Ahmad Z, et al.47 showed that xylanase reached it maximum production of 44U/ml at 72h by A. niger using sugarcane bagasse. Notably, Okafor UA, et al.48 studied the effect of different carbon sources on the production of xylanase by A. niger. When wheat bran was used, the maximum production of xylanase was obtained at 96h, giving rise to 6.47U/ml whereas 0.95U/ml was attained when sugarcane pulp was used. In addition, the study further indicated that sawdust and oat spelt xylan gave the maximum peak of xylanase activity of 0.65U/ml and 0.80U/ml at 120h, respectively. In conclusion, there was different carbon sources of agricultural residuals applied for the xylanase production by different Aspergillus strains.
Incubation temperature is an important factor that affects the outcome of xylanase activity. In this study, incubation temperature of A. brasiliensis was set at 30°C. Aspergillus strains such as A. niger, A. carbonarius and A. ochraceus were best grown at 30°C as proven by CabreraI HP, et al.49 They observed that when the temperature was over 35°C, the growth of fungi was lengthier. Furthermore, Sood M50 showed that the optimum growth of A. umbrosus was also occurred at 30°C. They reported that the fungi possessed a narrow range of temperature tolerance because of fungal sporulation took place in a narrower range of temperatures than vegetative growth. Figure 7 shows the spore count of A. brasiliensis during production of xylanase under batch submerged fermentation after inoculated with an optimum standard inoculum size of 1× 106 spores. Notably, Qinnghe C, et al.51 indicated that the best range for the initial inoculums size was between 106 and 107 spores per ml. From Figure 7, there was an increase of approximately 1300% of spore biomass observed in the bioreactor. The maximum spore count of A. brasiliensis in shake flask culture was 1.08× 109 ±0.017spores/ml while 1.513× 1010 ±0.035spores/ml was observed from bioreactor at 48h. This significant increase of biomass production explained the reason of higher xylanase activity in bioreactor. The correlation between xylanase activity and spore count is shown in Figure 8. From the graph, there was an increase in spore count and xylanase activity observed from 24h to 48h. Further incubation after 48h showed the reducing trend of both xylanase activity and spore count. This clearly indicated that the spore production was directly influenced the enzyme activity. In other words, more spore production produced higher xylanase activity by A. brasiliensis.
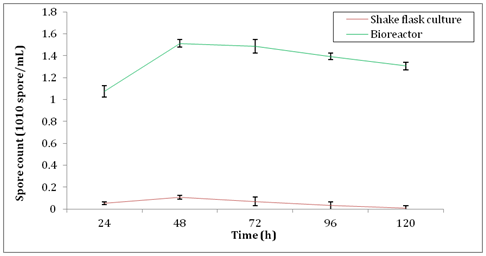
Figure 7 Spore count of A. brasiliensis during production of xylanase in shake flask culture and bioreactor.
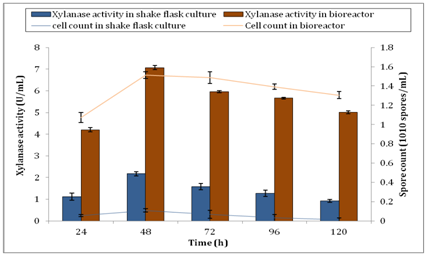
Figure 8 Correlation between xylanase activity and cell count by A. brasiliensis for shake flask culture and bioreactor.
Another factor that affected the xylanase activity and biomass production in this study was detected to be the medium pH. Figure 9 shows the pH profile of medium during production of xylanase by A. brasiliensis in shake flask culture. The highest xylanase activity was attained at pH 6.0 during 48h of batch submerged fermentation in shake flask culture. Takahashi Y, et al.52 stated that the xylanase activity produced by Aspergillus sp. at the mildly acidic pH range between pH 4 to pH 6. Medium pH decreased over time due to the production of acid such as citric acid by the fungi during batch fermentation process.53 Although A. brasiliensis favors the condition of slight acidic, as fermentation prolonged, the pH reached beyond the tolerated pH and thus, causing acid stress to the growth and reduced the biomass production and hence the xylanase activity.40 As for bioreactor, the medium pH was maintained and thus, the production of xylanase by A. brasiliensis was anticipated to be higher compared to shake flask culture.
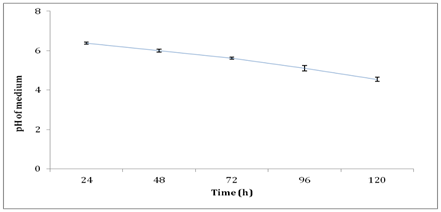
Figure 9 pH profile of medium during production of xylanase by A. brasiliensis in shake flask culture.
In Figure 10, it shows the soluble proteins produced by A. brasiliensis during batch submerged fermentation in shake flask culture and bioreactor. The highest protein concentration produced by shake flask culture was 1.679±0.118g/ml. Production of protein concentration in bioreactor was higher than shake flask culture with the peak of 4.760±0.043g/ml at 48h. The specific enzyme activity for xylanase in this study at 48h was 1.295U/g for shake flask culture and 1.486 U/g for bioreactor, respectively.
Purity of enzymes is very important during enzymes extraction. It can be identified through calculation of the specific enzyme activity. The specific enzymes activity by B. subtilis in shake flask culture was 1.557U/g and 2.167U/g in bioreactor, respectively. For A. brasiliensis, the specific enzymes activity of 1.295U/g and 1.486U/g was obtained from shake flask culture and bioreactor, respectively. Based on these results, B. subtilis produced purer xylanase per gram protein in this study. Therefore, B. subtilis is one of the preferable producers of xylanase and barley husk is a potential agricultural residual to be utilized as carbon source using shake flask culture and bioreactor in this study.
In conclusion, B. subtilis and A. brasiliensis show potentials of being xylanase producers. Major enzymes in world market including xylanase, protease and amylase are produced mainly by these two microorganisms. In the present study, xylanase reached its maximum activity of 13.069±0.193U/ml and 7.074±0.089U/ml by B. subtilis and A. brasiliensis in bioreactor, respectively. These results clearly showed that the application of bacteria and fungi in the producing of commercial enzymes are possible especially from Bacillus and Aspergillus strains. In addition, carbon source plays an important role in the production of microbial xylanase. The cost of producing enzymes is very expensive. Synthetic carbon source is very outlays and this would increase the production cost. To produce cost effective enzymes, agricultural residuals are introduced in many industries. Agricultural wastes are ample with nutrients and sugars content that are suitable to utilize by microorganisms during fermentation process. Besides that, they are cheap and easily available in large quantity. Each day, tons of agricultural wastes are produced in the world that contributed to pollution as a result of indecorous treatment. Therefore, by using agricultural wastes as the carbon source, they are not only aid to reduce the cost but also help in preventing pollutions. Consequently, wheat bran and barley husk had been chosen as the main carbon source in this study. They showed great potentials by producing significant amount of xylanase activity by B. subtilis and A. brasiliensis, respectively. Nonetheless, the growth of microorganisms and production of the enzymes are affected by the growth conditions such as temperature and medium pH. Bacteria have broad range of heat tolerance compared to fungi and thus, the enzymes produced by bacteria exert higher thermo stability. Hence, when fungi are used as the enzymes producer, temperature should be adjusted to accommodate the growth of fungi to produce the optimum growth and to maximize the enzymes activity. In this case, 30°C is the optimum temperature for A. brasiliensis. Research on the finding of the optimum growth condition for a particular microorganism strain that is labeled as the potential enzymes producer is very crucial for enhancing the optimum production of enzymes. Larger amount of enzymes are produced in a shorter period of fermentation time under the optimum conditions would further reduce the production cost in the industry point of view. In a nutshell, the optimized agricultural residual and optimum growth conditions are two important keys for the future of enzymes production in order to cope with the increasing demands of enzymes especially xylanase in worldwide market. As a recommendation, more studies on the potential enzymes producing strains, optimization of carbon sources and growth conditions would be very beneficial as they are responsible not only to reduce the production cost but also assist in preventing the long-term pollutions to the surrounding.
None.
The author declares no conflict of interest.

©2016 Ling. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.