Journal of
eISSN: 2572-8466


Review Article Volume 11 Issue 4
Department of Bioengineering, University of California, USA
Correspondence: Bill Tawil, Department of Bioengineering, UCLA School of Engineering, 420 Westwood Plaza, Room 5121, Engineering V. P.O. Box: 951600, Los Angeles, CA 90095-1600, USA, Fax +(310) 794-5956
Received: August 01, 2024 | Published: August 16, 2024
Citation: Lakshmi M, Tawil, Bill. A review on coronary artery disease. J Appl Biotechnol Bioeng. 2024;11(4):113-128. DOI: 10.15406/jabb.2024.11.00368
Coronary Artery Disease (CAD) is currently one of the most widespread and deadly conditions in the world. Occurring due to atherosclerosis of the coronary artery and possessing a plethora of risk factors, the disease boasts an extremely large market size which is projected to continue increasing well into the future. Therefore, it is of utmost importance to conduct further research into the management of CAD and utilize this knowledge to develop new products and procedures to decrease its pervasiveness. The biological characteristics of healthy and diseased tissue, global and US market sizes, prevalence, trends, existing treatments, and products in clinical trials relating to CAD are discussed in detail below.
Keywords: coronary artery disease, heart disease, cardiovascular disease, ischemic heart disease, atherosclerosis
CAD is reported to be the primary cause of mortality in the United States (US), and the third leading cause of death in the world, and therefore presents a pressing issue.1 Claiming over 9 million lives in 2019 and affecting nearly 200 million people around the world, the disease’s prevalence is set to only grow as the number of individuals possessing its risk factors increases.2,3,4
CAD takes place when the heart does not receive sufficient amounts of nutrients and oxygen from the coronary arteries which supply it with blood.2 In a healthy heart, the coronary arteries provide the organ with oxygenated blood directly from the aorta.5 The right coronary artery and left coronary artery work together to deliver essential materials which aid the heart’s performance of various functions such as contraction of muscle and propagation of electrical signals.5 However, in a diseased heart, this ability is diminished due to the occurrence of atherosclerosis, or the thickening of artery linings.6 This is primarily caused by the deposition of foam cells in the artery’s subendothelial lining.7 The severe accumulation and calcification of plaque in CAD leads to reduced patency, or a decreased ability of blood to travel through the vessel.6 The resulting risk of blood clot formation also plays a part in increasing the progression of the disease.7 This narrowing of the coronary arteries can be detected via various tests such as Electrocardiograms, Echocardiograms, and X-rays.7,8
The most common indicators of CAD are chest pain, shortness of breath, and fatigue.2 However, as the condition of the heart worsens over a long period of time, more severe complications such as arrhythmias, ischemic stroke, and myocardial infarction, also known as a heart attack, may occur.2,9 However, these severe conditions can also occur acutely, as the plaque may rupture and form a blood clot.9 CAD is often preventable and usually develops over a period of decades; therefore early diagnosis and treatment is necessary in order to promote appropriate management.1 Various risk factors for atherosclerosis, and therefore CAD, exist - some of which include high cholesterol, Diabetes, Chronic Kidney Disease, hypertension, and smoking.2 The risk of developing CAD also increases if someone has a family history of heart disease, as well as with age.2 Additionally, CAD rates are significantly higher in the male population, where rates increase after age 45.9 For women, rates increase after age 55.9 CAD is prevalent in many groups, such as white, African American, Hispanic, and Asian / Pacific Islander populations.10 Interestingly, different subgroups of one population may experience varying rates of disease incidence and mortality.10 Similarly, even in the same country, CAD prevalence can vary based on state / region.10 Another factor which influences the incidence of CAD is formal education, with increasing education having a correlation with decreased rates of the disease.10
The global market size for CAD is extremely large, accounting for a total of $26.3 billion in spending in 2023.6 Taking into account projected Compound annual growth rates of 10.2% and 8.86%, the market size is expected to increase to $28.97 billion in 2024 and $45.23 billion in 2030.11,12 In the US alone, more than 18.2 million individuals have been diagnosed with CAD, leading to the loss of over 375,000 lives.1,13,14
Treatment for the disease include drug-based strategies such as blood thinning medications, statins, Beta blockers, ACE Inhibitors, ARBs, Nitrates, Calcium channel blockers, and Diuretics.15 Many surgical interventions can also be pursued, including PCI, CABG, and heart transplants.15 Finally, devices such as stents, coronary balloons, and vascular grafts can be implanted in order to aid with treatment of CAD.15 Specifically, synthetic, biologic, and hybrid vascular grafts offer various advantages and disadvantages.16 Additionally, various other products and procedures are being developed, with several currently undergoing clinical trials.17–26
Healthy tissue
The coronary arteries are responsible for blood flow to the heart.5 Through this action, they are able to nourish the organ with necessary nutrients in order to ensure that it can pump blood throughout the body.5 These arteries diverge from the aorta, the point where freshly oxygenated blood leaves the heart, and are able to grow deep into the heart muscle to supply blood to the entire organ.5 The two main arteries - the right and left variations - branch off into smaller arterioles that surround the heart, similar to how regular blood vessels become smaller capillaries.5 Blood flow to the right atrium and right ventricle is supplied by the Right Coronary Artery (RCA), while blood flow to the left atrium and left ventricle is supplied by the Left Coronary Artery (LCA), as shown in Figure 1.5 The RCA also brings blood to the major electrical nodes of the heart, the Sinoatrial and Atrioventricular nodes.5 These are responsible for initiating and maintaining the rhythm of heart signals and contraction.5 Finally, both the RCA and LCA work in tandem to supply the interventricular septum, or the wall which separates the ventricles, with adequate blood flow.5
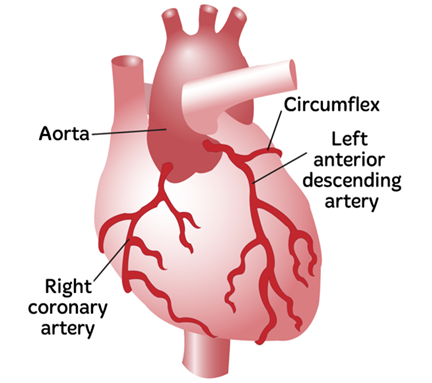
Figure 1 Diagram of Coronary Arteries, including the RCA and LCA.77 The image shows the heart with the Right Coronary Artery and Left Coronary Artery marked.
The coronary arteries are about 3 to 4 mm in diameter, and are composed of 3 layers called the Tunica intima, Media, and Adventitia.5 The Tunica intima is the innermost layer of the artery.5 The Media is the elastic inner portion and aids blood pressure maintenance.5 The outer layer or Adventitia plays a role in supplying the heart with nutrients through the use of smaller blood vessels.5
Several actions can be taken in order to maintain healthy coronary arteries and subsequently a healthy heart.5 A healthy diet, in combination with regular exercise and cessation from smoking can play a large role in reducing one’s risk for coronary artery-related diseases.5 Reduction of alcohol consumption can help as well.5
Diseased tissue
In CAD, due to various factors such as an unhealthy diet and lack of exercise, fatty material called plaque accumulates in blood vessel walls and ultimately reduces blood flow to the heart.7 CAD is characterized by the initial formation of ‘fatty streaks,’ which are deposits of macrophages known as foam cells.7 Macrophages are a key player in the immune system and help maintain healthy body function by assisting in the cleanup of unwanted pathogens and debris.27 When these macrophages arrive at a damaged portion of the coronary artery, they ingest low-density-lipoprotein particles and become foam cells.7 Foam cells then become lodged in the subendothelial layer of the blood vessel lining and begin decreasing the patency of the vessel.7 This process is accelerated by the activation of T cells and the release of growth factors as well.7 As the accumulation of foam cells grows and stabilizes over time, the area becomes calcified, resulting in further blockage of blood flow.7
If the plaque breaks or becomes dislodged, it can cause thrombosis or clotting through the exposure of Tissue Factor.7 This results in further obstructions in the artery, as shown in Figure 2.7 Once the artery becomes 90% blocked, angina (chest pain caused by reduced blood flow to the heart) can occur at a resting state.7,27
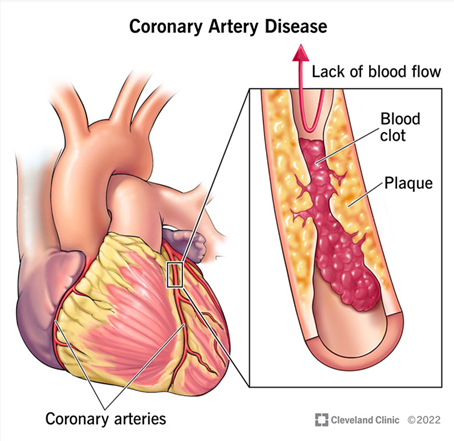
Figure 2 2022 Diagram of blocked Coronary Artery.76
The presence of plaque in the coronary arteries - and therefore a diagnosis of CAD - can be determined through use of an Electrocardiogram (EKG), Echocardiography, Stress test, Chest X-ray, Cardiac catheterization, Blood work, and Nuclear scanning.28,7 In addition to these tests, CAD patients can be identified by the specific symptoms they often present to the hospital with.2 These indicators are most often angina, shortness of breath, and fatigue, all caused by the diseased tissue’s inability to pump adequate blood to the body.2 It is important to keep in mind that these symptoms may be indicative of several outcomes such as arrhythmias, ischemic stroke, and myocardial infarction.2,4 A sedentary lifestyle, unhealthy diet, high cholesterol levels, Diabetes, Chronic Kidney Disease, hypertension, and smoking may all advance the progression and severity of CAD.2 A family history of heart disease and old age play a major role as well.2
Market size and trends
Global market size
CAD is the third most severe cause of death worldwide.3 In 2019, this disease claimed responsibility for the highest amount of mortality globally, accounting for nearly 9.1 million deaths.5 It is estimated that around 200 million people exist who have CAD worldwide - 110 million men and 80 million women.5 Due to the high prevalence of individuals with CAD and its severity, the market size for the disease is quite large, and is only expected to grow in upcoming years.6 $26.3 billion was spent for CAD treatment in 2023, and the market size is anticipated to increase to $28.97 billion in 2024, as shown in Figure 3A.6 With a compound annual growth rate (CAGR) of 10.2%, the market size is expanding rapidly as rates of CAD diagnosis increase.6 Other factors which contribute to this expansion are improved treatment options, an increase in sedentary lifestyles, and an increasingly aging population.6 Another estimate claims that with a CAGR of 8.86%, the CAD market size will reach $45.23 billion in 2030, as shown in Figure 3B.7 North America and Europe have the highest influence on the CAD market size.8 However, the Asia-Pacific is anticipated to see the highest growth until 2028 with the highest CAGR.8
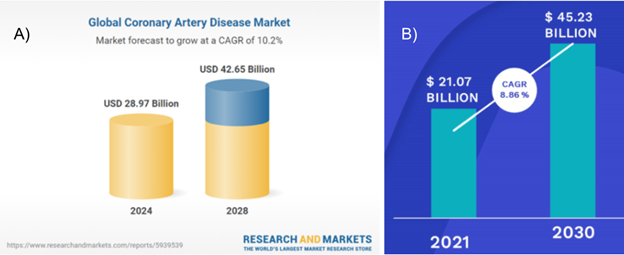
Figure 3 A) Global market size for CAD in 2023, and expected market size for CAD by 20286 B) Global CAD market predictions for 2021 - 2030.7
The image shows a diseased coronary artery, in which blood flow is restricted because of plaque buildup.
US market size
CAD is the highest cause of death in the US - in 2021, CAD was the cause of death for over 375,000 people in the US alone.3,9 In the US, there are 18.2 million individuals above the age of 20 who have CAD.10 As shown in Figure 4, the percentage of individuals with CAD varies by state, with populations in the midwest being more likely than others to have the disease.11 CAD is believed to affect nearly 5% of adults over 20, 10.9% of adults over 45, and 17% of adults over 65.9,11 However, a disproportionate amount of death - nearly 80% - is observed in patients who are 65 years or older.9
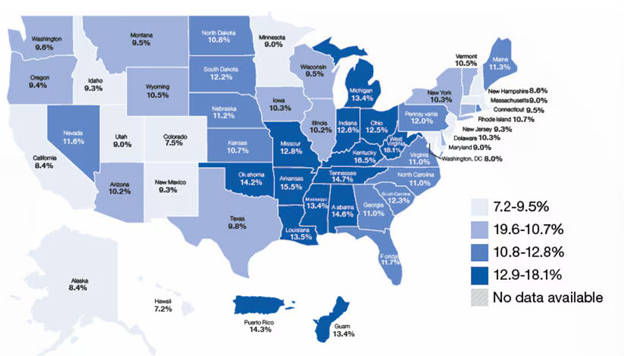
Figure 4 Adults in the US aged 45 and older who have CAD, reported by state.11
The image shows two predictions for the growth of the market size for CAD.
Prevalence
CAD is more prevalent in men than women, with males making up about 5 million of total deaths in 2019, while women made up about 4.2 million deaths.5 Providers regularly diagnose 13.6% of men and 8.4% of women with CAD.11 In addition, it is most prevalent in white, African American, Hispanic, and Asian / Pacific Islander populations, with the rates of CAD being at 11.4%, 10%, 8.8%, and 6.3%, respectively.11 In addition, black individuals are more likely to not only have but also die from a heart attack when compared to white individuals, which is related to CAD.12 Interestingly, younger Hispanic women are more likely to die from a heart attack in comparison to young Hispanic men, which contradicts the general increased susceptibility of men to CAD.12 Since the Asian / Pacific Islander population consists of many different subgroups, CAD mortality rates contain variations depending on subgroup, as shown in Figure 5.13 Formal education also played a role in the incidence of CAD, as adults with more schooling were less likely to develop the disease - 10.9% of those with a high school diploma versus 16.5% of those without.11
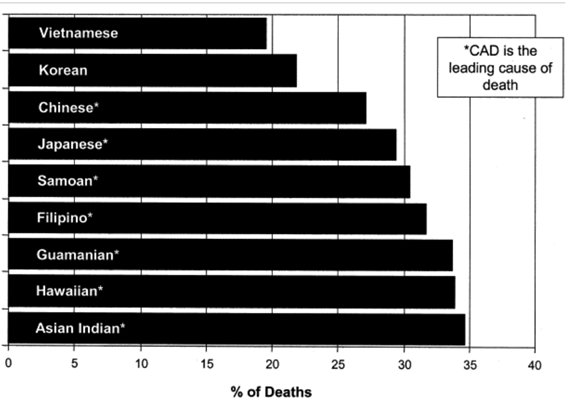
Figure 5 Percentage of deaths in US Asian / Pacific Islander populations due to CAD.13 The image shows the prevalence of CAD in various populations in the US.
Emerging trends
In 2020, 1 billion people were aged 60 years or over, and this number will likely increase to 2.1 billion by 2050.14 Since old age is a major risk factor for CAD, the incidence of the disease and therefore its market size is expected to increase with this trend.1,6,14 In addition to this demographic shift, there is an increase in spending towards the development of new technologies which can aid in the diagnosis and treatment of CAD.6 Due to these factors, the market size for CAD is projected to reach $42.65 billion by 2028.6 When observing trends for Cardiovascular diseases as a whole, the prevalence of the risk factors and therefore the disease itself is expected to increase from 2025 to 2040, stagnate from 2040 to 2050, and begin rising again in 2050, as shown in Figure 6.15
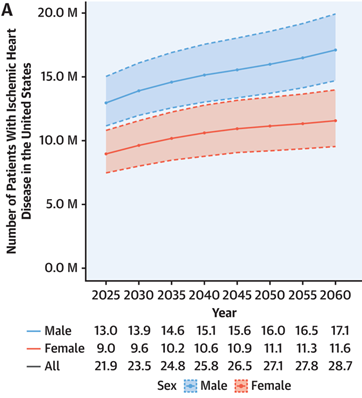
Figure 6 Estimated Population of Individuals with Ischemic Heart Disease from 2025 to 2060.15
The image shows the predicted growth in the amount of people with CAD.
Existing products
Due to the wide incidence of CAD, numerous options exist which mainly aim to improve blood flow through the coronary arteries.16 Different treatment options may be recommended based on the severity of the disease and its progression.16 Some examples of current treatments for CAD include drug-based strategies, surgical procedures, device implantation, and tissue engineering treatments.16 These solutions are discussed in further detail below:
Drug-based strategies
Blood thinning medications
Blood thinning medications are often prescribed to decrease the viscosity of blood and promote increased flow through partially blocked arteries.16 This also helps prevent a complete blockage of the coronary artery by decreasing the risk of blood clotting.16 Some common blood thinning medications include Aspirin, Clopidogrel, Rivaroxaban, Ticagrelor, and Prasugrel.16
Statins
Statins are medications prescribed in order to reduce cholesterol levels.16 Since high cholesterol is a risk factor for atherosclerosis, focusing on the management of cholesterol, especially low-density lipoprotein cholesterol, is an appropriate CAD treatment strategy.2,16 Some commonly prescribed statins include Atorvastatin, Simvastatin, Rosuvastatin, Pravastatin, and Fluvastatin.1
Beta blockers, ACE inhibitors, and ARBs
Beta blockers, Angiotensin-converting enzyme (ACE) inhibitors, and Angiotensin-2 receptor blockers (ARBs) are commonly used as a treatment for high blood pressure or hypertension.16 Beta blockers can also provide the additional effect of helping reduce angina.16 Some examples of Beta blockers include Atenolol, Bisoprolol, Metoprolol, and Nebivolol.16 Some examples of ACE Inhibitors are Ramipril and Lisinopril.16
Nitrates
Nitrates are often prescribed as vasodilators, meaning that they work by promoting the widening of blood vessels.16
Calcium channel blockers
Calcium channel blockers are often prescribed due to their dual effects of decreasing blood pressure as well as promoting vasodilation.16 Some common calcium channel blockers include Amlodipine, Verapamil, and Diltiazem.16
Diuretics
Diuretics promote the excretion of water and salt from the body.16
Surgical procedures
Surgical procedures are often considered if the patient is unresponsive to medication, or if their symptoms are too severe to be treated with medications alone.16 A coronary angiogram is usually performed to determine whether these procedures are necessary.16
PCI
Percutaneous coronary intervention (PCI, also known as Coronary angioplasty, Percutaneous transluminal coronary angioplasty, and Balloon angioplasty) is a surgery in which a balloon is inserted into the artery in order to widen the blocked area.16 Stents may then be used to hold the blood vessel open and prevent further thinning of the artery.16 Various types of stents exist, such as a drug-eluting stent, which mainly focuses on the release of medications to prevent blockage of the vessel.16
CABG
Coronary artery bypass graft (CABG, also known as Bypass surgery, Heart bypass, and Coronary artery bypass surgery) is a procedure in which an alternate blood vessel is grafted onto a vessel in order to connect the aorta to a piece of the coronary artery which is downstream of the blocked area.16 The replacement artery used for CABG can be an artery from the patient’s own leg, chest, or wrist, or a tissue engineered Vascular Graft can be used.17,18 During this procedure, the patient is usually connected to a heart-lung bypass machine, which assumes the function of pumping blood while the surgery is in progress.18 Various types of this procedure exist, such as the Off-pump coronary bypass (OPCAB), which does not require the use of a heart-lung machine and is therefore less invasive.5,18 Another example is the keyhole surgery, which involves performing the surgery through smaller incisions rather than being an open-heart surgery.18
Heart transplant
A total heart transplant is only pursued as a last resort when a patient appears to be unresponsive to medications and alternative surgical procedures.16
Devices
Stents
Stents are small mesh tubes which are commonly utilized to keep passages such as airways or blood vessels open.19 Stents used for treatment of the coronary artery are often classified as bare metal or drug-eluting.19 Bare metal stents do not have further embellishment of their core metal structure.19 However, drug-eluting stents contain an external layer surrounding the metal core, which usually carries medications that function to reduce the risk of restenosis (narrowing of the blood vessel for multiple instances).19 The outer layer of these stents may be designed to degrade over time.19
Drug-eluting stents are the most popular choice for use in the coronary artery and therefore procedures such as PCI.19 Some common Drug-eluting stents are Medtronic’s Onyx Frontier DES and Resolute Onyx DES, Boston Scientific’s ELUVIA DES, Promus PREMIER, and Synergy / Synergy Megatron DES, Concept Medical’s Abluminus DES+, Abbott’s XIENCE Skypoint and Sierra DES, and Terumo Aortic’s Relay Pro, Relay Plus, and Thoraflex Hybrid devices.20–34 A summary table containing information about these devices’ specifications, characteristics, and materials is shown below.
|
Company |
Product Name |
Specifications |
Details |
Materials |
|
Medtronic |
Onyx Frontier DES20
|
2 / 2.25 / 2.5 / 2.75 / 3 / 3.5 / 4 / 4.5 / 4 mm diameter 8 / 12 / 15 / 18 / 22 / 26 / 30 / 34 / 38 mm length |
Low crossing profile Flexibility Dual-flex balloon High deliverability |
Cobalt alloy shell Platinum-iridium core Zotarolimus drug (reduces neointimal growth) BioLinx polymer (rapid healing) |
|
Resolute Onyx DES21
|
2 / 2.25 / 2.5 / 2.75 / 3 / 3.5 / 4 / 4.5 / 5 mm diameter 8 / 12 / 15 / 18 / 22 / 26 / 30 / 34 / 38 mm length
|
Designed for complex PCI |
Cobalt alloy shell Platinum-iridium core Zotarolimus drug BioLinx polymer |
|
|
Boston Scientific |
ELUVIA Drug-Eluting Vascular Stent System22,23
|
6 / 7 mm diameter 40 / 60 / 80 / 100 / 120 / 150 mm length |
Controlled drug release |
Nickel Titanium alloy (Nitinol) PBMA PVDF-HFP Paclitaxel (reduce restenosis)
|
|
Promus PREMIER24
|
2.25 / 2.5 / 2.75 / 3 / 3.5 / 4 mm diameter
8 / 12 / 16 / 20 / 24 / 28 / 32 / 38 mm length
|
Good deliverability Good flexibility |
Platinum Chromium Everolimus
|
|
|
Synergy and Synergy Megatron25,26
|
Synergy 2.25 / 2.5 / 2.75 / 3 / 3.5 / 4 / 4.5 / 5 mm diameter Megatron 3.5 / 4 / 4.5 / 8 / 12 / 16 / 20 mm length |
Albuminally coated Polymer degrades naturally over time |
Everolimus |
|
|
Concept Medical |
Abluminus DES+27
|
2.25 / 2.5 / 2.75 / 3 / 3.5 / 4 / 4.5 / 5 diameter 8 / 12 / 16 / 20 / 24 / 28 / 32 / 36 / 40 / 44 / 48 / 52 length |
Edge coating to deal with restenosis Albuminal and fusion coating (drug release) |
Cobalt Chromium Alloy L605 Sirolimus Biodegradable polymer matrix |
|
Abbott |
|
4.5 / 5 mm diameter 12 / 15 / 18 / 23 / 28 / 33 mm length |
Larger expansion |
Anti-thrombotic Fluoropolymer Everolimus |
|
|
2,25 / 2.5 / 2.75 / 3 / 3.25 / 3.5 / 4 mm diameter 8 / 12 / 15 / 18 / 23 / 28 / 33 / 38 mm length |
profile |
||
|
TERUMO Aortic |
Relay Pro32
|
22 - 46 mm diameter Bare Stent 90 - 250 mm length Non-Bare Stent 99 - 259 mm length |
Reduced profile delivery Wide range of options |
Woven polyester |
|
Relay Plus33
|
22 - 46 mm diameter 100 - 250 mm length |
Minimum access vessel trauma Wide range of sizes |
Woven polyester Hydrophilic coating |
|
|
Thoraflex Hybrid34
|
D1 Graft 22 / 24 / 26 / 28 / 30 / 32 mm diameter D2 Graft 24 / 26 / 28 / 30 / 32 / 34 / 36 / 38 / 40 mm diameter 240 mm tensioned length 100-150 mm nominal length |
Coronary balloons
Coronary balloons are often used in procedures such as PCI in order to compress plaque buildup into the walls of the vessel and improve blood flow through it.35 The balloon is often attached to a catheter, which is a thin tube that can be easily slipped into blood vessels.35 Once the balloon is positioned at the site of blockage, it is inflated, which causes the surrounding plaque to be pushed against the blood vessel wall.35 Some common coronary balloons include Medtronic’s Eupora Semi Compliant Balloon Catheter, NC Euphora Noncompliant Balloon Catheter, Sprinter Legend 1.25 Balloon Catheter, Sprinter Legend RX Semicompliant Balloon Catheter, NC Sprinter RX Noncompliant Balloon Catheter, and NC Stormer OTW Noncompliant Balloon Catheter, and Boston Scientific’s Athletis Ultra-High Pressure Balloon, Charger 0.035” Balloon Dilatation Catheter, Mustang 0.035” Balloon Dilatation Catheter, Peripheral Cutting Balloon Microsurgical Dilatation Device for Hemodialysis Access Management, Ranger Paclitaxel-Coated PTA Balloon Catheter, and Sterling and Sterling SL 0.018” Balloon Dilatation Catheter.36–47 A summary table of commonly used coronary balloons, along with their manufacturers, specifications, and further details is provided below
|
Company |
Product Name |
Specifications |
Details |
|
Medtronic |
Euphora Semicompliant balloon catheter36
|
1.5 / 2 / 2.25 / 2.5 / 2.75 / 3 / 3.25 / 3.5 / 3.75 / 4 mm diameter 6 / 10 / 12 / 15 / 20 / 25 / 30 mm length |
Decreased crossing profile Good deliverability |
|
|
NC Euphora Noncompliant Balloon Catheter37
|
2 / 2.25 / 2.5 / 2.75 / 3 / 3.25 / 3.5 / 3.75 / 4 / 4.5 / 5 mm diameter 6 / 8 / 12 / 15 / 20 / 27 mm length |
High RBP Good deliverability |
|
|
Sprinter Legend 1.25 Balloon Catheter38
|
1.25 mm diameter 6 / 10 / 15 / 20 mm length |
Low crossing profile |
|
Sprinter Legend RX Semicompliant Balloon Catheter39
|
1.25 / 1.5 / 2 / 2.25 / 2.5 / 2.75 / 3 / 3.25 / 3.5 / 3.75 / 4 mm diameter 6 / 10 / 12 / 15 / 20 / 25 / 30 mm length |
Low crossing profile Many sizes |
|
|
|
NC Sprinter RX Noncompliant Balloon Catheter40
|
2 / 2.25 / 2.5 / 2.75 / 3 / 3.25 / 3.5 / 3.75 / 4 / 4.5 / 5 mm diameter 6 / 9 / 12 / 15 / 21 / 27 mm length |
High pressure capability |
|
|
NC Stormer OTW Noncompliant Balloon Catheter41
|
2 / 2.25 / 2.5 / 2.75 / 3 / 3.25 / 3.5 / 3.75 / 4 mm diameter 6 / 11 / 14 / 16 / 18 / 21 / 26 / 31 mm length |
Controlled compliance High pressure capabilities |
|
Boston Scientific |
Athletis Ultra-High Pressure Balloon42
|
20 / 30 / 40 / 60 / 80 / 100 mm length |
Able to withstand high pressures and highly resistant lesions Low crossing profile |
|
|
Charger 0.035” Balloon Dilatation Catheter43
|
3 mm diameter 20 / 30 / 40 / 60 / 80 / 100 / 120 / 150 mm length 75 / 135 cm shaft |
Noncompliant Large size range |
|
|
Mustang 0.035” Balloon Dilatation Catheter44
|
3 mm diameter 20 / 30 / 40 / 60 mm length 40 / 75 / 135 cm shaft |
Longer lengths More powerful dilatation |
|
|
Peripheral Cutting Balloon Microsurgical Dilatation Device for Hemodialysis Access Management45
|
5 / 6 / 7 / 8 mm diameter 20 mm length 50 / 90 / 135 cm shaft |
Minimal vessel trauma
|
|
|
Ranger Paclitaxel-Coated PTA Balloon Catheter46
|
4 / 5 / 6 / 7 mm diameter 40 / 60 / 80 / 100 / 120 / 150 / 200 mm length |
DCB with low doses TransPax coating for transfer of drugs |
|
|
Sterling and Sterling SL 0.018” Balloon Dilatation Catheter47
|
2 mm diameter 20 / 30 / 40 / 60 / 220 mm length 90 / 150 cm shaft |
Low lesion profile |
Vascular grafts
Vascular grafts (also known as vascular bypass) are utilized in surgical procedures such as CABG in order to promote blood flow around a blocked region of the coronary artery, or to simply replace the damaged portion of the blood vessel.17,48,49 Many different materials can be used to create a Vascular graft, such as synthetic polymers, biologic polymers, or a combination of both types.48 Cells are often loaded onto the Vascular Grafts in order to promote the growth of new tissue around the graft.17 The different types of materials and cells in order to produce various types of tissue engineered Vascular Grafts are discussed in more detail below.
Synthetic grafts
Materials commonly used to produce synthetic tissue engineered Vascular Grafts are poly-L-lactide (PLLA), polylactic acid (PLA), poly-ε-caprolactone (PCL), polyglycolide (PGA), silicone, poly (lactide-co-glycolide) (PLGA), poly-4-hydroxybutyrate (P4HB), poly(glycolide/caprolactone) P(GA/CL), poly(lactide/caprolactone) P(LA/CL), poly(glycerol sebacate) (PGS), polyurethane (PU), poly (ester urethane) urea (PEUU), polyethylene terephthalate (PET), and polytetrafluoroethylene (e-PTFE).17,48 These synthetic grafts can be loaded with various cells such as autologous bone marrow-derived mononuclear cells (BM-MNCs), bovine smooth muscle cells (SMCs), human SMCs, bovine endothelial cells (ECs), bovine fibroblasts, murine stem cells, and human pericytes, or they can remain acellular.48
Biologic grafts
Materials commonly used to produce biologic tissue engineered Vascular Grafts are fibrin, silk fibroin, collagen, elastin, chitosan, and gelatin.48 These scaffolds can then be loaded with cells such as ovine dermal fibroblasts, bovine vascular SMCs, ovine bone marrow smooth muscle progenitor cells, ovine ECs, fibroblasts, porcine SMCs, porcine ECs, murine aortic SMCs, human umbilical vein SMCs, and murine vascular SMCs, or they can remain acellular.48 In addition to tissue engineered vascular grafts, a blood vessel can be obtained from a patient’s leg, or an alternative chest artery.48 In addition to these natural polymers, decellularized matrices can also be utilized to create a Vascular Graft.48 Some examples of matrices which can be decellularized for use in a graft include human iliac veins, porcine arteries, canine carotid arteries, human umbilical veins, porcine small intestinal submucosa, and human amniotic membrane.48 These decellularized matrices can be loaded with cells as well, such as autologous SMCs, autologous ECs, autologous bovine ECs, canine bone marrow-derived SMCs, canine bone marrow-derived ECs, human umbilical cord vein ECs, and fibroblasts, or they can remain acellular.48
Hybrid grafts
Some grafts exist which use a mix of synthetic and biologic components in order to produce a hybrid tissue engineered Vascular Graft.48 Some combinations of materials used include PCL with spider silk and chitosan, PCL with synthetic elastin, PCL with PU-collagen composites, gelatin-vinyl acetate copolymer, and PU with PEG-fibrin.48 Similar to synthetic and biological grafts, hybrid grafts can also be loaded with cells such as bovine SMCs, bovine fibroblasts, bovine ECs, murine SMCs, and murine smooth muscle progenitors, or they can remain acellular.48
A summary table of various Vascular Graft products, including manufacturer, picture of product, product specifications, details, and material types, is included below.
|
Company |
Product Name + Picture |
Measurements |
Details |
Material |
|
Gore Medical |
GORE-TEX Vascular Grafts50
|
Standard-walled Ringed 12 / 14 / 16 / 20 mm internal diameter 30 / 20 cm ringed section length 30 / 20 cm standard length Thin-walled 4 mm internal diameter 80 cm standard length Thin-walled Removable Ringed 10mm internal diameter 70 cm ringed section length 70 / 80 standard length |
No preclotting Resist dilation Resist spread of infection Thrombectomy safety
|
Synthetic ePTFE |
|
|
GORE-TEX Stretch Vascular Graft51
|
Large Diameter 12 / 14 / 16 / 18 / 20 / 22 / 24 mm internal diameter 40 / 20 cm standard length Standard-walled Axillobifemoral Removable Ringed 8 mm internal diameter Fully ringed ringed section 70 / 40 / 90 cm standard length Standard-walled 5 / 6 / 7 / 8 / 10 mm internal diameter 4-6 / 4-7 mm internal diameter (tapered) 40 / 80 / 20 / 30 / 40 / 50 / 70 / 90 / 45 / 55 / 48 cm standard length Thin-walled removable ringed 5 / 6 / 8 mm internal diameter 30 / 40 / 60 / 70 cm ringed length 40 / 50 / 70 / 80 / 100 cm standard length |
No preclotting Resist dilation Resist spread of infection Thrombectomy safety
|
Synthetic ePTFE |
|
|
GORE-TEX Stretch Vascular Graft for Vascular Access52
|
Standard-walled fixed ringed dialysis 6 mm internal diameter 5 cm ringed section length 45 cm standard length Standard-walled ringed 6 mm internal diameter 10 / 5 cm ringed section length 20 cm standard length Thin-walled removable ringed 6 mm internal diameter 10 cm ringed section length 10 cm standard length Standard-walled 6 / 7 / 8 mm internal diameter 4-6 / 4-7 mm internal diameter (tapered) 10 / 20 / 30 / 40 / 50 cm standard length Standard-walled ringed 6 mm internal diameter 10 cm ringed section length 20 cm standard length Standard-walled ringed dialysis 6 mm internal diameter 5 cm ringed section length 45 cm standard length Thin-walled removable ringed 6 mm internal diameter 10 cm ringed section length 10 cm standard length |
Rapidly-tapering designs for potential complications with arterial steal syndrome Supporting rings allow for support and tight loop conformations |
Synthetic ePTFE |
|
|
GORE INTERING Vascular Graft53
|
Thin-walled stretch 6 / 7 / 8 mm internal diameter 40 / 60 / 80 cm radial supported length 40 / 60 / 80 cm standard length Standard-walled stretch 6 mm internal diameter 4-6 / 4-7 mm internal diameter (tapered) 5 / 10 / 38 / 40 cm radial supported length 20 / 40 / 45 cm standard length |
Soft, strong Minimize trauma to tissue Cutting or suturing at any point |
Synthetic Reinforced ePTFE |
|
LeMaitre |
Artegraft Collagen Vascular Graft54
|
4 / 5 / 6 / 7 / 8 mm inner diameter 15 / 30 / 35 / 40 / 45 / 50 cm minimum length |
Outperformed ePTFE in clinical studies Increased thrombus formation Decreased patency rates |
Biologic Bovine carotid artery |
|
|
AlboGraft Polyester Vascular Grafts55
|
Knitted straight 6 / 7 / 8 / 10 / 12 / 14 / 16 / 18 / 20 / 22 / 24 mm diameter 15 / 30 / 40 / 60 / 100 cm length Knitted bifurcated 12x6 / 12x7 / 14x7 / 14x8 / 16x8 / 16x9 / 18x9 / 18x10 / 20x10 / 20x11 / 22x11 / 24x12 mm diameter 50 cm length Knitted straight with removable support 6 / 7 / 8 mm diameter 40 / 60 / 80 cm length Woven straight 6 / 7 / 8 / 10 / 12 / 14 / 16 / 18 / 20 / 22 / 24 / 26 / 28 / 30 / 32 / 34 / 38 mm diameter 15 / 30 / 40 / 60 cm length Woven bifurcated 12x6 / 12x7 / 14x7 / 14x8 / 16x8 / 16x9 / 18x9 / 18x10 / 20x10 / 22x11 / 24x12 mm diameter 50 cm length |
Good sealing Compression / kinking resistance
|
Hybrid Polyester Bovine skin collagen
|
|
|
LifeSpan ePTFE Vascular Grafts56
|
Regular wall-straight 6 / 7 / 8 mm diameter 10 / 20 / 50 / 80 cm length Thin wall-straight 6 / 7 / 8 / 10 mm diameter 20 / 50 / 80 cm length Regular wall-external spiral support 6 / 7 / 8 / 10 mm diameter 50 / 80 cm length 50 / 80 cm spiral support Thin wall-external spiral support 6 / 7 / 8 / 10 mm diameter 50 / 80 cm length 50 / 80 cm spiral support Regular wall-center spiral support 6 mm diameter 50 cm length 10 cm spiral support Thin wall-center spiral support 6 / 7 / 8 mm diameter 50 / 80 length 30 / 50 spiral support Regular wall-stepped 4-7 mm diameter 50 cm length Regular wall-stepped with center spiral support 4-7 mm diameter 50 cm length 10 cm spiral support Regular wall-quick tapered 4-7 mm diameter 50 cm length Regular wall-quick tapered with center spiral support 4-7 mm diameter 50 cm length 10 cm spiral support |
Removable external support More than 15 years in clinical use *not sold in the US |
Synthetic ePTFE |
|
TERUMO Aortic |
Gelsoft Plus57
|
6 / 7 / 8 / 10 / 12 / 14 / 16 / 18 / 20 / 22 / 24 mm diameter 12.5 / 15 / 25 / 30 / 40 / 50 / 60 cm length |
Decreased possibility of graft infection |
Hybrid Polyester Gelatin
|
|
|
Gelweave58
|
21 / 24 / 26 / 28 / 32 / 34 / 36 / 38 / 42 / 44 mm diameter 15 cm length |
Design specifically for aortic arch anatomy |
Hybrid Woven polyester Gelatin
|
|
BARD |
Impra Carboflo Vascular Grafts59
|
4mm diameter 10 cm length |
Reduces risk of early graft failure
|
Synthetic Dacron PTFE |
Products in clinical trials
Various clinical trials are currently underway in order to advance the development of tissue engineered Vascular Grafts, as well as stents.60–70 In addition, comparisons of various procedures and their effects on CAD are underway.65,66 A table containing current and completed clinical trials relating to products for CAD treatment is provided below.
|
Title |
Product / Procedure of Interest |
Status |
Information |
|
|
A Pilot Study Investigating the Clinical Use of Tissue Engineered Vascular Grafts in Congenital Heart Surgery60 |
Graft |
Completed December 17, 2009 - March 2, 2018 |
Focus on graft failure rates and related mortality 4 participants |
|
|
Two-Year Study of the Safety and Efficacy of the Second-Generation Tissue-Engineered Vascular Grafts (TEVG-2)61 |
Graft |
Recruiting July 13, 2020 - August 2027 (estimated) |
Focus on graft-related mortality rates 24 participants |
|
|
Comparison of PCI in Native Arteries Versus Bypass Grafts in Patients with Prior CABG (CAPTAIN)62 |
Graft |
Completed October 1, 2019 - May 1, 2022 |
Focus on effects of native vessel PCI in comparison with graft PCI 1491 participants |
|
|
Percutaneous Coronary Intervention of Native Coronary Artery Versus Venous Bypass Graft in Patients with Prior CABG63 |
Graft |
Recruiting January 22, 2019 - June 30, 2027 (estimated) |
Focus on effects of native vessel PCI compared to bypass graft PCI 584 participants |
|
|
PANTHER Study of Terumo Aortic Knitted and Woven Grafts, and Cardiovascular Patches64 |
Graft |
Recruiting February 17, 2021 - January 2033 (Estimated) |
Focus on collecting data on the safety of Terumo products 2000 participants |
|
|
Hybrid Revascularization Versus Coronary Artery Bypass Grafting65 |
Hybrid Coronary Revascularization (HCR) |
Not yet recruiting January 2025 - December 2040 (estimated) |
Focus on hybrid coronary revascularization (combination of PCI and CABG) in comparison to CBG alone 1200 participants |
|
|
HCR vs. CABG Study66 |
HCR |
Completed August 2018 - July 7, 2018 |
Focus on whether outcomes of hybrid coronary revascularization in comparison to outcomes of CABG 75 participants |
|
|
iFR Guided Coronary Artery Bypass Grafting Surgery67 |
CABG |
Recruiting April 25, 2022 - August 31, 2026 |
Focus on observing outcomes of CABG when intra-coronary physiology measurements are made vs. angiographic imaging is used to determine which vessels to bypass 100 participants |
|
|
Outcomes of Drug Coated Balloon Angioplasty, A UK Real Life Experience From 2009 to 201568 |
Balloon Angioplasty |
Active, not recruiting September 11, 2017 - January 1, 2027 |
Focus on outcomes of Drug Coated Balloon Angioplasty treatment in comparison to Drug Eluting Stent insertion treatment. 1000 participants |
|
|
BIOHELIX-1 Bare Metal Stent Study69 |
Stent |
Completed November 2012 - November 2017 |
Focus on recording collecting data on safety of BIOTRONIK Pro-Kinetic Energy stent 329 participants |
|
|
Everolimus Stent in Patients with Coronary Artery Disease (CAD)70 |
Stent |
Completed March 2003 - June 2010 |
Focus on comparison of first-generation DES to second-generation DES, specifically on release of drug Everolimus 600 participants |
|
CAD is one of the most common and deadly diseases in the world, affecting nearly 200 million people and accounting for nearly 9.1 million deaths.5 It occurs due to the accumulation of plaque in the coronary arteries which nourish the heart, a process also known as atherosclerosis.1,2 It is prevalent in many populations such as whites, blacks, Hispanics, and Asian / Pacific Islanders, and generally affects more men than women.5,11 Individuals suffering from high cholesterol, high blood pressure, Diabetes, and Chronic Kidney Disease possess a higher risk of developing the disease - therefore, the incidence of CAD is closely tied to the incidence of other conditions.1 With an estimated market size of $28.97 Billion in 2024 and a CAGR of 10.2%, the demand for CAD treatment will only increase, and experts believe the market may be as large as $42.65 Billion in 2028.6 Popular treatments to treat CAD can be classified as drug-based strategies, surgical procedures, insertion of devices, and vascular grafting.16 Drug-based strategies include the use of blood thinners, statins, Beta blockers, ACE Inhibitors, ARBs, Nitrates, Calcium channel blockers, and Diuretics.16 Popular surgical procedures done to treat CAD are PCI and CABG - a heart transplant can also be done as a last resort.16 Some common devices that can help with promoting blood flow through the coronary arteries are stents, coronary balloons, and vascular grafts.17–49 Specifically, vascular grafts can be made from synthetic and biologic materials, or a mix of both.48 Finally, many products designed to treat CAD are currently in clinical trials, and data exists for those who have previously completed them as well.60,61 Overall, CAD is a very widespread disease with many potential treatments - however, as the incidence rates are expected to increase, further research and development of new products is necessary.1,15,16
Lakshmi Mohan would like to thank Professor Bill Tawil for his aid with the organization of the review, as well as the valuable lectures on tissue engineering which also greatly assisted in the creation of this paper.
Authors declare that there is no conflict of interest.
None.

©2024 Lakshmi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.