eISSN: 2574-9838


Research Article Volume 9 Issue 3
Attending Physician Specialist in Physical and Rehabilitation Medicine, Rehabilitation Unit, Traumatologic-Orthopedic Center, University Hospital of Careggi, Italy
Correspondence: Giuseppe Falcone, Attending Physician Specialist in Physical and Rehabilitation Medicine, Rehabilitation Unit, Traumatologic- Orthopedic Center University Hospital of Careggi, Florence, Italy
Received: July 15, 2024 | Published: August 14, 2024
Citation: Falcone G. Efficacy of an intensive multimodal rehabilitation program in adult patients affected by complex regional pain syndrome type 1 (CRPS 1): a randomized controlled trial. Int Phys Med Rehab J. 2024;9(3):91-97 DOI: 10.15406/ipmrj.2024.09.00381
Introduction: The Complex Regional Pain Syndrome (CRPS) type 1 is a complex nosological entity, mostly with post-traumatic genesis (osteo-myo-articular traumas of various nature, in particular bone fractures, especially in the case of fractures treated conservatively with immobilisation in plaster), characterised by intense painful joint and peri-articular symptoms that can be extremely disabling, with pain frequently refractory to the usual therapeutic strategies - pharmacological and otherwise - and with consequent important algo-functional limitations and sometimes severe reduction in the person's quality of life. The aim of the present clinical study is to verify the role of Physical and Rehabilitation Therapy and the related Individual Rehabilitation Project in the treatment of patients suffering from Complex Regional Pain Syndrome type 1 (CRPS 1) and in particular to evaluate the efficacy of a specific intensive and multimodal Rehabilitation Program in adult patients suffering from CRPS 1.
Materials and methods: An open-label randomised controlled clinical trial (RCT) was conducted between September 2018 and May 2024 at the SODc Riabilitazione CTO of the AOU Careggi Hospital in Florence on 36 patients suffering from Complex Regional Pain Syndrome type 1 (diagnosed by the Budapest Criteria) in the acute - sub-acute phase (i.e. within 3 months of symptom onset) with severe/disabling pain symptoms (i.e. spontaneous pain intensity in the affected limb ≥ 50 mm on the Visuo-Analogue Scale [VAS] and CRPS Severity Score ≥ 5, [Italian version modified by Zyluk, J Hand Surg Br 2003]). The study was conducted on two parallel groups - study arms: the study group, consisting of 18 patients, was treated by pharmacological therapy with Neridronate ev. according to the therapeutic scheme of Varenna et al. in association with an Individual Rehabilitation Project according to a specific intensive multimodal rehabilitation program. The control group, on the other hand, also consisting of 18 patients, was treated with the same drug therapy but without rehabilitation treatment.
Results: With regard to the primary endpoint of the study, i.e. pain intensity measured by means of a visuo-analogue scale (VAS) in a range from 0 mm (no pain) to 100 mm (maximum pain), the results show a more significant reduction in the mean VAS in the study group compared to the control group. The collected data were then statistically analysed and comparisons between the means were performed by analysis of covariance (ANCOVA). The p-values obtained were in all cases below the initially established statistical significance level of α=0.05. These results are in line with those obtained for the secondary end-points, i.e. the McGill Pain Questionnaire Short-form (SF-MPQ), the CRPS Severity Score, the functional assessment questionnaires for the affected limb (Quick DASH for the upper limb and LEFS for the lower limb), the SF-12 questionnaire for the assessment of the patient's quality of life: the latter highlight a significant improvement of the "functional" endpoints in the study group compared to the pre-treatment values and above all highlight the existence of a relevant difference in the comparison between the two groups of patients, with evidence of greater therapeutic efficacy in terms of recovery of function and improvement of quality of life in the study group compared to the control group.
Conclusion: The results of this study suggest that Physical and Rehabilitation Therapy plays a pivotal role in the treatment of patients with Complex Regional Pain Syndrome type 1 and that the Individual Rehabilitation Project based on the proposed Rehabilitation Program is safe and effective for these patients, resulting a component of primary importance in the therapeutic management of complex regional pain syndrome.
Keywords: complex regional pain syndrome, CRPS 1, rehabilitation
The Complex Regional Pain Syndrome (CRPS) type 1, traditionally named and known in the medical scientific community as Algodystrophy or Algodystrophic Syndrome (AS), is a complex, multisymptomatic nosological entity, characterised primarily by intense joint and peri-articular pain symptoms associated with loco-regional vasomotor disturbances and presenting a non-metameric distribution, most often localised to the distal segments of a limb (the hand or foot).1
CRPS type 1 has incidence rates described in the scientific literature of between 5 and 26 people per 100,000 per year2,3 particularly after fracture and surgical trauma the incidence of CRPS type 1 in the various studies is between 30% and 40% of all cases overall.4
It is therefore a pathology whose occurrence is far from infrequent, also and above all in Rehabilitation Medicine, and which, moreover, as reported by numerous Authors, is on the whole under-diagnosed and in many cases belatedly diagnosed.5 All this often results in a chronicisation of the clinical picture characterized by persistent pain, joint stiffness and kinesiophobia.
The pathogenetic mechanisms that trigger and feed the syndrome are still only partially known: primum movens at the origin of the algodystrophic syndrome is considered to be an inadequate response to osteo-myo-articular damage associated with abnormal post-traumatic inflammation.6 The assumption that the predominant problem is a dysfunction of the sympathetic system and that the syndrome evolves according to fixed stereotyped stages is now obsolete and has been progressively abandoned.7,8
According to recent etiopathogenetic theories, the syndrome is rather characterised by a complex interaction of numerous peripheral and central pathogenetic mechanisms, which are summarised below on the basis of the evidence that has emerged from studies in recent years: -aberrant inflammatory phenomena and neuroflogosis sustained by neuropeptides and pro-inflammatory cytokines; -vasomotor dysfunction and microcirculation damage resulting in hypoxia and loco-regional tissue acidosis; -peripheral nociceptive sensitisation; -sympathetic nervous system dysfunction and pathological sympathetic ‘coupling’ with peripheral adrenergic hypersensitivity; -central sensitisation and maladaptive neuroplasticity (with secondary cortical reorganization); -protective disuse (sometimes linked to the onset of a ‘neglect-like’ syndrome); -aberrant post-traumatic healing process.9,10
Beyond the possible pathogenic noxae and mechanisms involved, various Authors emphasise that the primum movens and common denominator of the syndrome is represented by a maladaptive response to a trauma - or in any case to a local damage of various nature and origin, not always identified and identifiable: the characteristics of the traumatic/stressful events, individual genetic factors and epigenetic mechanisms that condition the phenotypic response to the histolesive phenomenon interacting with each other give rise in the individual to a variable response to a trauma, which may be adaptive and lead to recovery, or maladaptive leading in some cases to the pathology defined as algodystrophy or Complex Regional Pain Syndrome.1,9,10,1
The clinical picture of the syndrome, although characterized by polymorphous manifestations, is predominantly defined - especially in the initial stages of the pathology - by intense pain (which is generally the predominant symptom and for which the patient turns to the doctor) with joint localization or at least district localization at the level of a limb with notable functional impotence and the possible presence of hyperalgesia (pain disproportionate to the nociceptive stimulus), hyperpathy (painful sensation that persists when the algogenic stimulus ceases) and allodynia (painful perception of normally non-algogenic stimuli), but also of local swelling (oedema is significant in many cases) with associated vasomotor alterations and alterations in skin trophism and skin adnexa.9
At the diagnostic level, the internationally recognized and validated clinical criteria for the diagnosis of CRPS type 1 that are most widely used in the clinical setting are those formulated in Budapest in 2007 at a consensus conference promoted by an IASP task force of experts (Table 1). These criteria are based on the finding of signs and symptoms located in the sensory, vasomotor, sudomotor and motor-functional spheres associated with the absence of an alternative diagnostic interpretation.12
|
A. The patient presents continuous pain disproportionate to the triggering event |
|
|
B. The patient has at least two or more signs in each category |
|
|
C. The patient reports at least one symptom in three or more categories |
|
|
D. No other diagnosis is able to justify signs and symptoms |
|
|
Categories |
Signs (examination objective) |
|
Sensory |
Allodynia (to light touch and/or hot-cold and/or deep pressure and/or joint movement) |
|
Hyperalgesia (at the pinprick) |
|
|
Vasomotor |
Hyperesthesia Asymmetry to thermo touch and/or alterations or asymmetry in the color of the skin (> 1° C) |
|
Sweating/ Edema |
Edema and/or alterations or asymmetry of sweating |
|
Tissue motility/trophism |
Reduction of the range of motion and/or motor dysfunction (weakness, tremor, dystonia) and/or Trophic variations(hair, nails, skin) |
Table 1 Budapest criteria (version translated into Italian)
In clinical practice in Physical Medicine and Rehabilitation, it is not uncommon to see patients who present the symptoms described above and who suffer from pain that is out of proportion to the causative event, poorly responsive to commonly used analgesic and anti-inflammatory drug therapy, and persistent even long after the traumatic event and/or surgery. The physiatrist is generally called upon because the patient, in addition to presenting a history of pain with the above-mentioned characteristics possibly associated with vasomotor-trophic alterations, also manifests important functional limitations that translate into difficulties in activities of daily living and consequent reduction - sometimes severe - of the person's quality of life.
CRPS type 1 is therefore a potentially disabling morbid entity, which has a high functional and social impact (including the related employment implications for affected patients). Timely diagnosis and early treatment of CRPS type 1 are of primary importance to prevent permanent functional limitations of the affected limb and to improve the patient's quality of life: only an early diagnosis of the syndrome in its early stages can in fact allow effective treatment, in which pharmacological therapy, although indispensable, must be associated with other non-drug therapeutic strategies.13
Beyond these assumptions widely recognized in scientific literature, however, there are still no standardized and univocally accepted protocols for the therapeutic management of patients with algodystrophic syndrome. Indeed, CRPS type 1 still remains a difficult condition to treat even in its early stages, both because of the numerous etiopathogenetic mechanisms involved and because of the syndrome's pathomorphological variability.1,11
This complexity of therapeutic approach also applies to the rehabilitation management of CRPS patients, which still represents a challenge that is as difficult as it is fascinating for all specialists in the field.
In the multiplicity of rehabilitation strategies and techniques proposed, there is on the whole a relative lack of high- grade evidence regarding the most effective and appropriate ones in CRPS type 1 patients and rehabilitation protocols that are valid in terms of clinical efficacy and effectiveness and reproducible have not yet been univocally defined.11, 14
What emerges from the above is the need to identify shared diagnostic, therapeutic and also rehabilitation pathways for patients suffering from CRPS type 1, based on scientific evidence and clinical ‘best practice’ and that could be useful to guide the clinician in his or her daily clinical practice.
It is in this perspective that has been developed the present study focusing on Rehabilitation in patients suffering from Complex Regional Pain Syndrome (CRPS) type 1.
The aim of this study is to define a specific Rehabilitation Program for patients suffering from Complex Regional Pain Syndrome type 1 in the acute-subacute phase (within 3 months of the onset of symptoms) that can provide the Physiatrist with a basis for drawing up, prescribing and implementing the Individual Rehabilitation Project, customized and ‘tailor-made’ for each patient suffering from the syndrome in its initial phases, which are decisive for the functional outcome. The above-mentioned rehabilitation protocol has been drawn up using three fundamental criteria as a basis: a) the relevant evidence currently available in the scientific literature; b) the consistency of the mechanism of action (scientifically proven) and of the therapeutic rationale of the proposed Physical Medicine and Rehabilitation techniques and strategies with respect to recent acquisitions on the pathogenetic mechanisms of Complex Regional Pain Syndrome type 1; c) the preliminary clinical results obtained in our experience and clinical practice.
Assess if, how and to what extent the rehabilitation treatment proposed in association with the already validated pharmacological therapy (intravenous neridronate according to the scheme of Varenna15 affects the therapeutic results in patients suffering from Complex Regional Pain Syndrome type 1 in the acute-subacute phase (within 3 months of the onset of symptoms) compared to the above-mentioned pharmacological background therapy alone.
The evaluation described above was carried out by a randomized controlled clinical trial in which the research hypothesis is that there is a difference in terms of clinical and imaging therapeutic results and functional outcome between the treatment group (drug therapy + rehabilitation therapy) and the control group (drug therapy alone) within the previously identified study population (patients with CRPS type 1 in the acute-subacute phase), and in particular with evidence of greater therapeutic efficacy in the treatment group. The alternative hypothesis (null hypothesis) is instead the absence of difference between the two study groups or the presence of evidence of greater therapeutic efficacy in the control group.
Firstly it has been developed an intensive, multimodal Rehabilitation Program for CRPS 1 – described below -, which is specifically aimed at patients with Complex Regional Pain Syndrome type 1 (diagnosed by the Budapest Criteria) with severe/disabling pain symptoms such as to require a specialized and multidisciplinary rehabilitation approach. This rehabilitation program is not intended as a rigid and non-modifiable treatment protocol, but rather as a basis for the prescription and implementation of the Individual Rehabilitation Project in these patients.
The study population, as anticipated, consists of patients suffering from Complex Regional Pain Syndrome type 1 (diagnosed by the Budapest Criteria) in the acute-subacute phase (within 3 months from the onset of symptoms) with severe/disabling pain symptoms (spontaneous pain intensity in the affected limb ≥ 50 mm on the visual-analogue scale [VAS] and score ≥ 5 on the CRPS Severity Score [Italian version modified by Zyluk, J Hand Surg Br 2003]; therapeutic intervention in the initial phase of the pathology is crucial for functional recovery, since it represents the phase of the disease in which disability is most modifiable and rehabilitation intervention can positively influence the biological processes underlying recovery, containing and reducing the extent of algo-functional limitations.
The study was conducted on two groups - parallel study arms - from the above-mentioned patient population: The study group was treated by pharmacological therapy according to the therapeutic scheme of Varenna.15 (i.e. Neridronate 100 mg/8ml fl ev, 1 fl diluted in 500 ml of physiological solution administered ev in 2h [slow intravenous infusion], for a total cycle of 4 administrations [cumulative dose 400 mg], one every 3 days) in association with early Rehabilitation by means of a specific intensive and multimodal Rehabilitation Program. The Rehabilitation Program proposed and adopted in the study group includes 8 weeks of outpatient rehabilitation treatment, characterized by 3 rehabilitation sessions per week on alternate days for a total of 24 rehabilitation sessions. This programme, based on the association of therapeutic exercise and physical therapy - instrumental and non-instrumental - provides in particular the association of the following rehabilitation techniques and strategies: district-specific active and active-assisted segmental kinesiotherapy, hydrokinesiotherapy, ‘neuro-motor’ exercise (in particular graded motor imagery and mirror therapy), functional re-education of the affected limb (occupational therapy for CRPS 1 of an upper limb, walking re-education and weight-bearing exercises for CRPS 1 of a lower limb) instrumental physical therapy (pulsed electro-magnetic fields, antalgic and functional electrotherapy, water immersion ultrasound therapy of the affected segment), proprioceptive re-education and local skin desensitization techniques, ‘vascular’ physiotherapy (manual lymphatic drainage, vascular gymnastics, hydrotherapy/contrast baths, taping and/or local anti-oedemigenous bandages).
The control group, on the other hand, was treated with the same drug therapy but without rehabilitation treatment, on the contrary with the only additional prescription to the drug therapy of functional rest of the affected limb (i.e. limb raised in unloading during night rest, walking with the aid of Canadian crutches in the case of lower limb involvement, application of brace/splint in the case of the upper limb).
With regard to the study protocol, firstly were established eligibility criteria, those adopted were the following: (a) inclusion criteria = diagnosis of CRPS type 1 using the Budapest criteria, CRSP localized to a hand or foot, patient age ≥18 years and ≤70 years, disease duration ≤ to 3 months, spontaneous pain intensity in the affected limb ≥ 50 mm on the visuo-analogue scale (VAS) in a range from 0 mm (no pain) to 100 mm (maximum pain), CRPS Severity Score (modified from Zyluk, J Hand Surg Br 2003) with a score ≥ 5 (Table 2), MRI positive for signs of CRPS; b) exclusion criteria = CRPS type 1 not diagnosable on the basis of the Budapest criteria, CRPS in anatomical regions and articular districts other than the hand or foot, patient's age <18 years and >70 years, duration of disease >3 months, spontaneous pain intensity in the affected limb <50 mm on the visual-analogue scale (VAS), CRPS Severity Score <5, the presence of concomitant pathologies (cardio-vascular, respiratory, renal, hepatic, gastro-enteric, endocrine-metabolic, neurological, haematological, infectious) in the acute phase and/or of functional decompensation, patient suffering from a malignant neoplasm in progress, state of pregnancy, presence of absolute contraindications to physical therapies adopted in the rehabilitation program. The choice of the above-mentioned criteria was primarily oriented towards ensuring the homogeneity of the sample and its representativeness in relation to the patient population under study.
|
Symptoms or sign: clinical expression |
greater |
moderate |
minor or absent |
|
Pain (at rest) |
2 |
1 |
0.5 |
|
Reduction of digital flexion (apex-palm/sole distance >6 cm) |
2 |
1 |
0.5 |
|
Swelling |
1 |
0.5 |
- |
|
Alteration of skin temperature |
1 |
0.5 |
- |
|
Changes in skin color (rubor, pallor, cyanosis) |
1 |
0.5 |
- |
|
Sensory alteration (allodynia, hyperesthesia, hypoesthesia) |
1 |
0.5 |
- |
|
Sweating alterations (anhidrosis, hyperhidrosis) |
1 |
- |
- |
|
Pain and reduction of limb movement. proximal (e.g. shoulder) |
0.5 |
- |
- |
|
Alterations in the trophism of the skin appendages (nails, hair) |
0.5 |
- |
- |
Table 2 CRPS severity score, Italian version modified from Zyluk, J Hand Surg Br 2003
As part of the study design, were then defined the end-points to be used as criteria for evaluating and measuring the outcome of the patients and for quantitatively verifying and comparing the results obtained. In particular, the primary end-point was chosen as pain intensity to the previously described visual-analogue VAS scale, given that pain represents the main clinical expression and the most disabling symptom in the context of the syndrome, and as secondary end-points: - the McGill Pain Questionnaire Short-form (SF- MPQ), for a more complete and multidimensional assessment of pain in these patients; - the CRPS Severity Score, a score that quantitatively expresses the severity of the clinical picture; - the scores for the functional evaluation of the affected limb (the Quick DASH [Disabilities of the Arm, Shoulder and Hand questionnaire] questionnaire for the upper limb or the LEFS [Lower Extremities Functional Score] questionnaire for the lower limb); - the SF-12 questionnaire (12-Item Short Form Health Survey) to evaluate the quality of life perceived by the patient. All these assessment scales and questionnaires were administered to each patient enrolled in the study before randomization and the start of treatment (baseline – T0) and then at the clinical checks, planned and carried out according to the following follow-up time scheme: - 1st clinical check 40 days from the start of treatment (i.e. the first neridronate infusion in both groups) (T1), 2nd clinical check 60 days (2 months) from the start of treatment (T2), 3rd clinical check 120 days (4 months) from the start of treatment (T3), 4th and last clinical check 6 months from the start of treatment (T4). In the context of these controls, further evaluations and clinical tests were also carried out, such as the clinimetric evaluation (with goniometer) of the joint Range of Motion (ROM) of the involved region, anthropometry with tape measure for the evaluation of the edema, the examination of muscle strength of the main muscle groups of the affected limb. These clinical tests, despite not having been included among the end-points of the study and not having been analyzed (due to the absence of sufficient homogeneity at baseline to allow an accurate analysis), provided additional elements for a more complete clinical examination of the patient and above all they highlighted a trend of results in line with those of the other criteria and outcome evaluation measures obtained in the two groups. Furthermore, the patients recruited into the study underwent an imaging evaluation (X-ray and MRI of the anatomical district involved) before recruitment (T0 - baseline), and then with serial checks at 40 days, 90 days and 6 months from the beginning of the treatment.
In the present clinical study, conducted between September 2018 and May 2024 at the SODc Rehabilitation of the CTO (Orthopedic Trauma Center) of the Careggi University Hospital of Florence, 36 patients suffering from Complex Regional Pain Syndrome type 1 were recruited on the basis of the inclusion and exclusion criteria previously described. The average age of the patients is 54 years (extreme 27-65 years), 17 patient’s male, and 19 female. 20 patients had algodystrophy affecting hand, 16 patients affecting foot. In all patients, CRPS was of post-traumatic origin, with the following identifiable pathogenic noxae fracture of the distal epiphysis of the radius (Colles' fracture) treated with non-surgical reduction and immobilization in plaster/brace (12 patients), - fracture of the tarsus and/or metatarsal bone treated conservatively (9 patients), - fracture of the carpus and/or metacarpal treated conservatively (6 patients), - ankle sprain with associated ligament injury treated conservatively (5 patients), - ankle sprain in the absence of ligament injury (1 patient), - isolated fracture of the lateral malleolus of the ankle treated non-surgically (1 patient), - fracture of the phalanx of a hand treated conservatively (1 patient), - post surgical decompression of the carpal tunnel (1 patient); the onset of the syndrome following the aforementioned traumatic events occurred within a time range varying from a few weeks to a few months after the trauma. The 36 patients recruited into the study were randomized into two groups: the study group (drug therapy + physical and rehabilitation therapy) made up of 18 patients, and the control group (drug therapy + functional rest of the affected limb) also made up of 18 patients. The randomization procedure, aimed at removing systematic errors and allowing a balanced comparison between the two groups, was carried out using computer aid for the random assignment of each patient to one of the two groups of the trial. With regard to the operating methods adopted during the study, needs to be clarified that in the study group the patients began the specific rehabilitation program in all cases after the third administration of neridronate, thus resulting in the rehabilitation treatment being early, chronologically parallel and almost concomitant with respect to the pharmacological one. The data analysis was planned according to the intention to treat methodology, even if all patients completed the study without side effects. The statistical significance level α established before the study is 5% (i.e. p = 0.05). The data obtained were analyzed both through the use of descriptive statistics, with graphic representation of the data themselves and use of the averages of the variables under study in the two groups, and through two-tailed hypothesis testing statistical tests: in particular, given the small size of the sample, we opted for non-parametric statistical tests and specific tests for repeated measures. Changes in VAS (primary endpoint) were assessed using a repeated measures analysis of covariance (ANCOVA) model; the results of the other questionnaires and outcome indices, that are the McGill Pain Questionnaire Short- form (SF-MPQ), the SF-12 questionnaire (12-Item Short Form Health Survey), the CRPS Severity Score and the function evaluation questionnaires of the affected limb (Quick DASH or LEFS) were analyzed using repeated measures analysis of variance models (Friedman test - non-parametric equivalent of ANOVA for repeated measures). Comparisons of the clinical-functional parameters obtained through the rating scales were performed using the Wilcoxon rank sum test. Beyond the aforementioned statistical analyses, to complete the interpretation of the study data, an evaluation of the clinical relevance of the results obtained was carried out: this evaluation was possible by calculating the 95% confidence intervals (95% CI) of the data obtained and use of the minimal clinically important difference (MCID) of the study outcome indices.
As regards the primary endpoint, i.e. the intensity of pain measured using a visual-analog scale (VAS) in a range from 0 mm (no pain) to 100 mm (maximum pain), the average values of the VAS at T0 were almost identical in the two groups of patients, in particular in the study group the mean VAS at T0 was equal to 73.4 (with standard deviation, SD: 12.5) and the mean VAS at T0 in the control group was equal to 72.6 (with standard deviation, SD: 11.1). The mean VAS values at T1 were equal to 23.7 in the study group (with SD: 10.6) and 43.1 in the control group (with SD: 7.3), at T2 they were equal to 15 .4 in the study group (with SD: 8.4) and 42.8 in the control group (with SD: 8.2), at T3 they were equal to 14.8 in the study group (with SD: 9, 1) and 43.4 in the control group (with SD: 10.3), at T4 they were equal to 14.5 in the study group (with SD: 10.2) and 41.7 in the control group (with SD: 9.7). These values are summarized in the relative graphic (Graph 1).
The collected data were then statistically analyzed and comparisons between means were performed with analysis of covariance (ANCOVA). The p-values obtained were in all cases lower than the level of statistical significance initially established, equal to α=0.05. These results lead to the exclusion of the possibility that the differences in the results between the two groups are due to chance and also lead to rejecting the null hypothesis of the H0 study, i.e. the absence of difference in the therapeutic results between the two treatment groups and vice versa to accept the 'hypothesis H1, with evidence of greater effectiveness of the study group's therapy compared to the control group. These results are further explained through graphical representation (Graph 2).
In the context of the analysis of the data obtained, it was analyzed, beyond statistical significance, what was the actual clinical relevance of the differences in the two treatment groups. Taking into account the reduction in VAS and in particular the difference between the mean pre-treatment VAS value and the mean VAS value at the last follow-up in each of the two groups, the 95% confidence interval (95% CI) of the obtained values was calculated and compared with the minimally clinically important difference (MCID) of the VAS which - on the basis of what is reported in the scientific literature16,17can beconsidered equaltoadecreaseof25 mmofthescore.
In both groups of patients the therapy was clinically effective in terms of pain reduction, with a reduction in VAS on average greater than MCID. However, in the control group only the upper limit of the confidence interval goes beyond the MCID, which implies that not all patients will perceive clinically significative effects, while in the study group the 95% CI is well and completely above of the MCID, highlighting that the treatment will produce more relevant clinically effects perceived by all treated patients. These considerations reinforce what was previously stated, highlighting the far from negligible therapeutic impact of the rehabilitation program in addition to pharmacological therapy in the patient’s population under study (Graph 3).
The results of the multidimensional pain assessment using the McGill Pain Questionnaire Short-form (SF-MPQ) are in line with those obtained for the VAS (Graph 4).
The data deriving from the analysis of the CRPS Severity Score, a clinical index for the severity of the disease, also reveal significant differences in the outcome of the two treatment groups, with earlier and greater remission of clinical symptoms in the study group compared to the control group (Graph 5).
Furthermore, the results of the imaging control exams (X-ray and MRI of the affected region) also highlight a regression of the findings attributable to algodystrophy in all patients of both treatment groups, confirming its therapeutic efficacy on the natural history of CRPS 1.
Of great interest - especially from a physiatric perspective - are the results obtained through indexes for the functional evaluation of the affected limb, i.e. the Quick DASH (Disabilities of the Arm, Shoulder and Hand) questionnaire for the upper limb or the LEFS questionnaire (Lower Extremity Functional Scale) for the lower limb, as well as through an index assessing the quality of life and disability, i.e. the SF-12 questionnaire (12-Item Short Form Health Survey). These results are illustrated in the relative graphics (Graph 6&7).
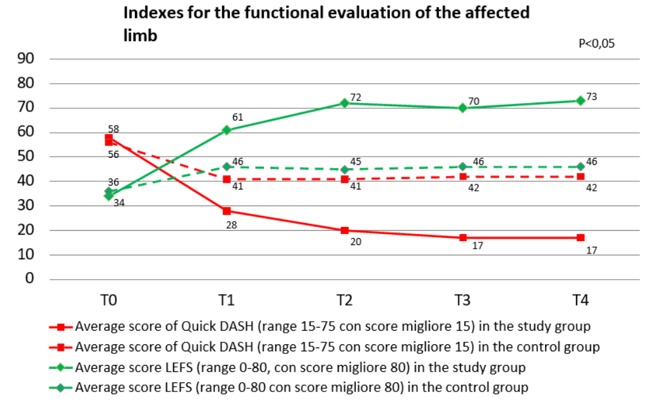
Graph 6 Time trend of the mean score of the functional outcome endpoints Quick DASH (for the upper limb) and LEFS (for the lower limb) in the two groups of patients.
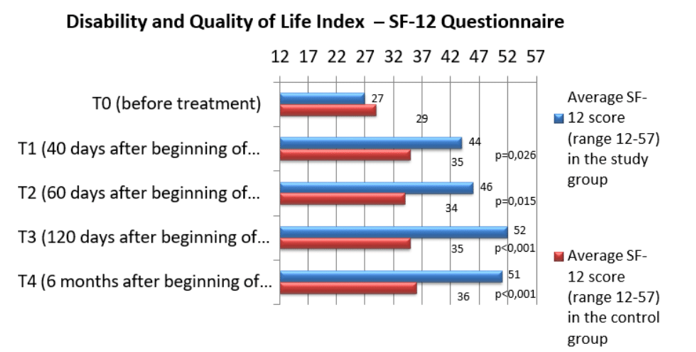
Graph 7 Comparison of the mean score of the SF-12 quality of life assessment endpoint in the two groups of patients.
SF-12 Domains: Limitations in physical activity; Limitations in social activities; Limitations in daily activities due to physical conditions; Physical pain; Vitality and energy; Mental health; Limitations in activities due to psychological/emotional disorders; General perception of health conditions.
The results highlight a significant improvement in the "functional" endpoints in the study group compared to pre-treatment values and above all highlight the existence of a significant difference in the comparison between the two groups of patients, with evidence of greater therapeutic efficacy in terms of recovery of function and improvement of quality of life in the study group compared to the control group.
The results obtained are encouraging and highlight that the rehabilitation treatment proposed in association with the pharmacological therapy already validated for the syndrome (intravenous neridronate according to the scheme of Varenna15 significantly affects the therapeutic results in patients affected by Complex Regional Pain Syndrome type 1 in the acute-subacute phase (within 3 months from the onset of symptoms) compared to pharmacological therapy alone, which is currently recognized as the treatment of choice for CRPS 1.
Specifically, on the basis of the analysis of the data obtained in this study it was possible to accept the research hypothesis that there is a difference in terms of therapeutic results and functional outcome between the treatment group (pharmacological therapy + rehabilitation therapy) and the control (only pharmacological therapy and functional rest of the affected limb) with evidence of greater efficacy in the treatment group, thus clarifying the role and clinical importance of the appropriate rehabilitation treatment in these patients.
The results obtained in the study also highlight some significant aspects relating to the Rehabilitation of Complex Regional Pain Syndrome type 1, aspects of great interest and that are still the subject of debate among specialists in the sector.
The data obtained highlight for example that the procrastination of the load and the protected load for long periods in patients affected by CRPS 1 of the lower limb and the corresponding functional rest in the case of CRPS 1 affecting the upper limb appear less effective compared to an early multimodal rehabilitation approach, in which early gradual loading and therapeutic exercise are significant elements for the remission of symptoms and recovery of function. A further example is represented by the role of instrumental physical therapy in patients suffering from CRPS 1: from the results of this study it emerges that the forms of physical therapy adopted appear useful for patients suffering from CRPS 1, also and above all from a perspective of synergistic therapeutic action with therapeutic exercise and with more recently introduced rehabilitation approaches in these patients (for example graded motor imaging and mirror therapy).
The limitations of the clinical study, of which the main ones are the small sample size and the absence of blinding (the study was not conducted blind), suggest however the need for further larger studies, possibly multicentric, to confirm what is here established.
Based on the results in this study it is possible to state that Physical and Rehabilitation Therapy plays a pivotal role in the treatment of patients suffering from Complex Regional Pain Syndrome type 1 and that the Individual Rehabilitation Project based on the developed intensive multimodal Rehabilitation Program is safe and effective for these patients, acting as a component of primary importance in the therapeutic management of the person affected by the syndrome.
None.
The author declares that there are no conflicts of interest.

©2024 Falcone. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.