eISSN: 2574-9838


Case Report Volume 9 Issue 3
1Department of Physical Medicine and Rehabilitation, Unidade Local de Saúde de Braga, Portugal
2Department of Neurology, Unidade Local de Saúde de Braga, Portugal
3Department of Physical Medicine and Rehabilitation, Unidade Local de Saúde do Alto Ave, Portugal
Correspondence: Gabi Almeida, Department of Physical Medicine and Rehabilitation, Unidade Local de Saúde de Braga,Hospital de Braga, Sete Fontes - São Victor, 4710-243, Braga, Portugal
Received: September 02, 2024 | Published: September 17, 2024
Citation: Almeida G, Soares I, Cruz C, et al. Bilateral lumbosacral plexopathy: A rare condition presenting as a diagnostic challenge. Int Phys Med Rehab J. 2024;9(3):107-110. DOI: 10.15406/ipmrj.2024.09.00383
Lumbosacral plexopathy (LP) is an uncommon condition. Its diagnosis may be challenging especially in the absence of evident structural causes and should be supported by lumbosacral imaging and neurophysiologic studies. LP is considered idiopathic when no causal factor is identified and it is thought to be immune-mediated. The authors report the case of a male with priors of radical prostatectomy and pelvic radiotherapy who presented the nosological triad of LP: lower limb progressive muscle weakness, sensory disturbances, and pain. Extensive investigation excluded different etiologies but no undoubtful causal conclusions were reached, raising the exclusion diagnosis of idiopathic LP as most likely. The patient was started on a rehabilitation program and several therapies, including corticotherapy, tocopherol, pentoxifylline, and hyperbaric oxygen therapy (the latter three targeting a less likely hypothesis of post-radiation LP), showing progressive improvement. Early intervention by a multidisciplinary team may have facilitated this favorable outcome. Despite all questions, the authors hope this report alerts to this rare entity.
Keywords: lumbosacral plexus, lumbosacral plexus/abnormalities, diagnosis, rehabilitation, hyperbaric oxygenation
CSF, Cerebrospinal fluid; CT, Computed tomography; CV, Conduction velocities; EBV, Epstein-Barr virus; EMG, Electromyography; HIV, Human immunodeficiency virus; HO, Hyperbaric oxygen; LL, Lower limb; LP, Lumbosacral plexopathy; MRC, Medical Research Council; MRI, Magnetic resonance imaging; PMR, Physical Medicine and Rehabilitation.
The lumbosacral plexus is a nervous network derived from the anterior rami of the L1-S4 nerve roots1 which provides sensorimotor innervation to the ipsilateral pelvic girdle and lower limb (LL).2 Lumbosacral plexopathy (LP) clinical features include muscle weakness and sensory disturbances in multiple contiguous lumbosacral nerve root distributions3 and depend on the level of involvement of the plexus.2
It comprises numerous etiologies1–3 and may be difficult to diagnose especially in the absence of evident structural causes.1,4 As such, its exact prevalence is unknown1 despite being uncommon4 and less frequently reported than brachial plexopathy.5 Table 1 describes some of the possible causes of LP.
|
Possible causes of lumbosacral plexopathy |
|
Local trauma |
|
o Accidents; intraoperative injuries; stretching or compression; sacral fractures; pregnancy or peripartum-related. |
|
Metabolic or autoimmune causes |
|
o Diabetes mellitus; sarcoidosis; connective tissue disorders.... |
|
Infectious causes |
|
o Gastrointestinal tract infections; urinary tract infections; spinal/vertebral infections; Human immunodeficiency virus (HIV) infection; Epstein-Barr virus (EBV) infection; Lyme disease; syphilis; tuberculosis; local abscesses.... |
|
Tumoral causes |
|
o Local malignant invasion; metastatic lesions; benign tumors; lymphomas..... |
|
Vascular causes |
|
o Retroperitoneal hematomas; arterial aneurysms; ischemia.... |
|
Radiation-induced |
|
o Radiation therapy of abdominopelvic malignancies.... |
|
Idiopathic |
Table 1 Possible causes of lumbosacral plexopathy (adapted1–3)
Legend: HIV, Human immunodeficiency virus; EBV, Epstein-Barr virus
LP is considered idiopathic when no causal factor is identified.3 The diagnosis itself can be challenging; apart from trying to determine the cause, it is important to rule out other differentials for paraparesis6 such as spinal cord injuries, motor neuron diseases, polyneuropathies, demyelinating diseases, and hereditary/familiar causes.
Pelvic magnetic resonance imaging (MRI) is the gold-standard test to assess the lumbosacral plexus1,3 and can help exclude some etiologies, being particularly useful to detect traumatic injury, compression, inflammation, or neoplastic infiltration.2,4 It is more sensitive than computed tomography (CT) scan to detect local malignancies.7
Neurophysiologic studies should also be conducted before the suspicion of LP and may help differentiate it from lumbosacral radiculopathies, peripheral neuropathies and motor neuron diseases.1-3 Electrodiagnostic findings can be variable and both nerve conduction studies and needle electromyography (EMG) should be performed to accurately diagnose LP and characterize its severity.3,4,8A thorough medical history check is crucial as it may hint on the etiology.1 Etiologic investigation should also include blood and cerebrospinal fluid (CSF) tests (Table 2).2
|
Laboratory tests |
|
|
Blood |
Cerebrospinal fluid |
|
· Complete blood count |
|
|
· Metabolic profile including glycosylated hemoglobin |
|
|
· Coagulation profile |
· Cell count |
|
· C-reactive protein, sedimentation rate |
· Cytology |
|
·Rheumatoid factor, antinuclear antibodies, anti-SSA/SSB, antineutrophil cytoplasmic antibodies |
· Glucose levels |
|
· Viral serologies |
· Protein levels |
|
· Screening for Lyme disease and syphilis |
· Viral panel |
|
· Angiotensin-converting enzyme |
· Microbiology panel, including antibody titer tests for Lyme disease |
|
· Serum protein electrophoresis, immunoglobulins |
· Angiotensin-converting enzyme |
|
· Paraneoplastic antibody panel |
|
Table 2 Laboratory tests – blood and cerebrospinal fluid (adapted1-3)
Regardless of the etiology, correctly diagnosing LP is of paramount importance to adequately manage this condition. By describing a case of idiopathic bilateral LP, the authors aim to raise awareness to this rare entity and to the importance of its accurate diagnosis.
We describe the case of a previously independent 74-year-old male with medical priors of type 2 diabetes mellitus, arterial hypertension, dyslipidemia, and previous smoking habits. He had no history of contact with COVID positive patients and had completed the respective vaccination schedule.
He was diagnosed with prostate adenocarcinoma and subject to radical prostatectomy in July 2019. In August 2021, he underwent salvation pelvic radiotherapy (total dose of 68Gy) due to biochemical recurrence of prostate-specific antigen associated with high-risk characteristics (Gleason score 8 and local invasion).
Three months after finishing radiotherapy, in November 2021, he began experiencing bilateral posterior lower leg pain that kept progressing in intensity and localization, ending up extending to the entire LL and lumbar region and affecting his functionality and quality of life. Afterwards, in December 2021, the patient started noticing progressive LL weakness and loss of independent gait. He also mentioned altered distal LL sensation and weight loss of over 10Kg in a few months.
The patient was then admitted to the Neurology ward for investigation in January 2022, presenting asymmetric flaccid paraparesis, with muscle strength globally grading 3 (Medical Research Council, MRC) in the right LL; in the left LL, he scored MRC grade 4 for the proximal segments and ankle plantar flexion, grade 2 for ankle dorsiflexion, and grade 1 for hallux extension. Right LL deep tendon reflexes and the left Achilles reflex were abolished, whereas the left patellar reflex was brisk; both plantar reflexes were normal. He also exhibited global LL muscle atrophy and needed crutches to walk, showing a more evident step page pattern on the left. Tactile and algic feet hyperesthesia was also noteworthy, with no proprioceptive deficits. Higher brain functions and cranial nerves changes, cerebellar signs, sensorimotor deficits on the upper limbs and face, fasciculations, or sphincter involvement were not identified.
Before this clinical picture, several diagnostic hypotheses were postulated and broad etiologic investigation was conducted, with a special focus on painful radiculopathies and neuropathies, myelitis/encephalitis, motor neuron diseases and myopathies. Exclusion of structural and paraneoplastic causes was also included. CSF analysis showed mild hyperproteinorachia; neoplastic cells and antibodies for autoimmune encephalitis were not detected.
Previous contact with Epstein-Barr virus (EBV) (detection of antibodies against EBV viral capsid and nuclear antigen) and hyperthyroidism stood out from the blood analysis. Antithyroid antibodies were negative and thyroid ultrasound was normal.
Nerve conduction studies revealed a significant decrease in the amplitudes and slight slowing in the conduction velocities (CV) of the motor action potentials of both tibial and fibular nerves. Reductions in the sensory amplitudes and CV of all the investigated LL nerves were also noticed. Needle EMG showed signs of active denervation of the investigated LL muscles. No myokymic discharges were registered. These results were suggestive of acute postganglionic sensorimotor injury with clinical expression in the LL.
Contrasted neuroaxis MRI ruled out space-occupying lesions and compressive myeloradiculopathies. New neoformative lesions with involvement of other organs and local tumor recurrence were also excluded by thoraco-abdominopelvic CT scan and pelvic MRI.
Lumbosacral plexus MRI showed signs suggestive of bilateral plexopathy, revealing edema of the plexuses’ roots extending to the emergence of the sciatic nerves. The patient was started on oral corticotherapy (after an initial intravenous bolus), and also gabapentin to address the neuropathic pain complaints. Although the investigation did not allow any definite conclusions to be drawn regarding the cause of the bilateral LP, the possibility of radiation-induced LP was considered based on the patient’s priors, and a trial of pentoxifylline and tocopherol was instituted.
The patient was also integrated into a rehabilitation program during hospitalization, aiming to improve LL muscle trophism and strength and including desensitization techniques and gait training. Sensory complaints and pain showed progressive improvement. However, at discharge in the end of January 2022, he maintained paraparesis with muscle strength globally grading MRC 4 except for grade 3 for ankle dorsiflexion bilaterally; he still needed assistive devices for gait and was oriented to maintain ambulatory rehabilitation.
Based on the same diagnostic rationale, the patient was referred to a Hyperbaric Medicine Centre and underwent a total of 60 sessions of hyperbaric oxygen (HO) therapy between March and May 2022. The patient presented progressive and significant motor recovery. At reevaluation in May 2022, he was capable of unassisted gait, maintaining a mild step page pattern bilaterally, and used a single crutch only in exterior environments with uneven ground. His thyroid hormones levels were back to normal.
For better understanding, Figure 1 depicts a timeline of events leading up to the end of the HO therapy sessions:
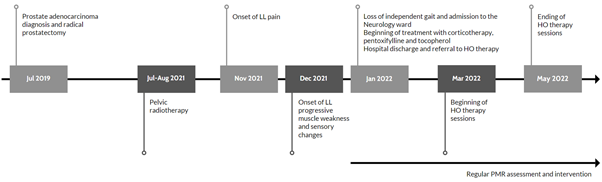
Figure 1 Timeline of events leading up to the end of the hyperbaric oxygen therapy sessions.
Legend: HO, hyperbaric oxygen; LL, lower limb; PMR, Physical Medicine and Rehabilitation
In September 2022, 10 months after the complaints started, control neurophysiologic studies revealed improvement in what concerns sensorimotor nerve CV and muscle recruitment. There were still some signs of active denervation, and neurogenic atrophy was more prominent in the L5 miotome bilaterally.
In the follow-up appointment at this time, no LL segmental motor deficits were detected, and the patient was able to walk independently without step page but unable to heel walk. He was independent in all activities of daily living. He maintained periodic rehabilitation and treatment with gabapentin, pentoxifylline and tocopherol, but had suspended corticosteroids and recovered his usual weight. Control MRI showed residual inflammatory changes of the lumbosacral plexuses.
New neurophysiologic studies were conducted in December 2023, about 2 years after clinical onset, maintaining gradual improvement particularly in the recruitment of the tested LL muscles, with no signs of active denervation.
This patient presented the nosological triad for LP described in the literature: LL progressive muscle weakness, sensory changes, and pain.1-4 As described, after excluding other differentials, bilateral LP diagnosis was supported by pelvic and lumbosacral MRI and neurophysiologic studies.
The patient’s history of diabetes mellitus could represent an etiologic factor. In fact, diabetic LP is a complication of type 2 diabetes mellitus typically associated to weight loss,2,9 which this patient experienced to a significant degree. However, diabetic LP is a unusual complication9,10 and the distribution of muscle weakness and pain tends to be more proximal, especially in the buttock area and posterior thigh,10 and symptoms usually begin unilaterally.2,9 The patient’s adequate glycemic control is also a point against this hypothesis, as it is more often associated with poor control.10
The weight loss could point towards neoplastic recurrence, though after its exclusion this sign might possibly be explained by the detected hyperthyroidism, which appears to have resolved spontaneously as shown in outpatient control blood tests. Vascular and tumoral causes were also ruled out through imaging. There was no history of accidents or intraoperative injuries. Stretching or compression of the plexuses during surgery were also unlikely due to the elapsed time.
As mentioned, based on the patient’s priors of pelvic radiation exposure and the presence of some risk factors for radiation-induced LP11 (advanced age, cardiovascular risk factors and total dose higher than 50Gy), this diagnosis could also be a possibility. Nonetheless, this is a rare complication of pelvic radiotherapy7 with clinical onset typically developing several years after exposure5,7 and classically described as progressive.2,12,13 The absence of myokymic discharges in the EMG also makes this hypothesis less likely, as they are highly suggestive of this entity, although not pathognomonic.12,13
Evidently, this case constituted a diagnostic challenge and no undoubtful causal conclusions were reached. As so, the hypothesis of idiopathic LP – an exclusion diagnosis4,14 – raised as most likely in the absence of other tacit explanations.
Idiopathic LP has similar clinical features to metabolic or autoimmune LP, presenting with acute to subacute onset of LL pain (which can be severe), followed by weakness and sensory changes.2 These manifestations can either be unilateral or bilateral and asymmetric.2
The pathophysiology of idiopathic LP is uncertain,5,14 but immune-mediated microvasculitis is thought to play a role.2,5,15 Viral infections may precede this disorder and an association with EBV has been suggested;4 this patient showed evidence of previous contact with EBV although infection timing and causality cannot be established.
Though it might be recurrent, the course of idiopathic LP is usually monophasic4,14 progressive recovery is expected but may not always be complete,14,16 especially regarding sensorimotor signs. Lumbosacral plexus MRI can show increased T2-weighted signal,14 possibly translating edema and inflammation.4,14
The treatment rationale for LP is to correct the cause whenever possible.2 In contrast, treatment of idiopathic LP should target the symptoms, with a special focus on pain management as neuropathic complaints are frequently key symptoms.1,5
Some studies point towards the use of corticosteroids with or without intravenous immunoglobulins;4,5,15,16 however, their effectiveness still lacks robust evidence.16 In this case, corticotherapy institution was meant to cover the inflammatory and possibly immune-mediated background.
As previously mentioned, considering the hypothesis of radiation-induced LP and although there are no completely proven effective therapies for it,3 the patient was also started on pentoxifylline and tocopherol based on available literature such as the report of Delanian et al.17 On the other hand, HO therapy, which is based on exposure to 100% fraction of inspired oxygen,18 has been presenting itself as a treatment option in cases of post-radiation complications, with some reports of efficacy in radiation-induced LP.19,20
In spite of diagnostic uncertainty, the patient showed progressive clinical improvement after the institution of the rehabilitation program and the several therapeutic weapons. This improvement was relatively rapid and started while he was still admitted and had yet to begin HO therapy. As such, its role in the clinical evolution might be questionable. Considering the idiopathic LP diagnosis, this clinical course would be the expected in the monophasic subtype.14 Early intervention may have played an important role in the favorable outcome as well.
Despite all questions, the authors hope this report may constitute an alert to this condition and wish to highlight the importance of a multidisciplinary approach, including the functionality-directed assessment of Physical Medicine and Rehabilitation and the role of physical therapy as a valuable adjunctive treatment modality.
None.
The authors have no conflicts of interest to declare.

©2024 Almeida, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 International Day of Persons with Disabilities takes place on December 03, 2025. The theme of this year is “Fostering disability-inclusive societies for advancing social progress” which is aimed to raise awareness about the challenges faced by persons with disabilities and to promote their full and equal participation in all aspects of society. To mark this occasion, IPMRJ invites researchers to submit their quality work and avail a 30% discount on publication.
International Day of Persons with Disabilities takes place on December 03, 2025. The theme of this year is “Fostering disability-inclusive societies for advancing social progress” which is aimed to raise awareness about the challenges faced by persons with disabilities and to promote their full and equal participation in all aspects of society. To mark this occasion, IPMRJ invites researchers to submit their quality work and avail a 30% discount on publication.