International Journal of
eISSN: 2574-8084


Case Series Volume 12 Issue 2
Clinical Specialist, Mindray Animal Medical, UK
Correspondence: Glavan Cristi, DVM MRCVS PhD, RCVS Recognised Specialist in Diagnostic Imaging, Clinical Specialist, Mindray Animal Medical, 5 Walton Crescent Stoke-on-Trent, UK
Received: March 28, 2025 | Published: April 15, 2025
Citation: Cristi G.Imaging findings and monitoring of a rare case of congenital intrahepatic bile duct ectasia (Caroli’s disease) in 2 cats from the same litter. Int Int J Radiol Radiat Ther. 2025;12(2):29-33. DOI: 10.15406/ijrrt.2025.12.00415
Congenital intrahepatic bile duct ectasia (Caroli's disease) results from in-utero malformation of the ductal plate.¹ It is a rare inherited disorder characterized by cystic dilatation (or ectasia) of the bile ducts within the liver.² Although well described in humans, in veterinary literature, only a few case reports have been published. Imaging modalities, ultrasonography, and computed tomography are used as a first-line approach in cases of abdominal pathology in cats. Although computed tomography (CT) is used to better describe possible abdominal pathology due to its excellent morphological resolution and ability to image different structures, ultrasonography is a baseline imaging modality for evaluating the feline and canine hepatobiliary system and monitoring the progression of a certain condition.
This report describes imaging findings (ultrasonography and computed tomography) and follow-up examinations of a congenital intrahepatic bile duct ectasia in 2 kittens from the same litter, with a coevolution of a pancreatic cyst in one of them.
Keywords: hepatobiliary, diagnostic imaging, cats
The ductal plate is a layer of hepatic precursor cells surrounding the portal venous branches and is the anlage of the intrahepatic bile ducts. The manifestation of ductal plate malformation depends on the level of the biliary affected, resulting in Caroli disease (the simple type) from the abnormal development of the large bile ducts and Caroli syndrome (the periportal type of Caroli disease).1,3 In Caroli syndrome, both the central intrahepatic bile ducts and the ductal plates of the smaller peripheral bile ducts are affected, leading to the development of fibrosis.1,4,5 Caroli disease and Caroli syndrome are rare autosomal recessive disorders with a slight female predilection. There is a high association with fibrocystic anomalies of the kidneys, which share the same genetic defect (PKHD1 gene), involvement of the liver, pancreas, and lungs is also well described in human medicine.6
Differentiation from obstructive biliary disease needs to be made to rule out possible secondary dilatation of the bile ducts.7 Causes of obstruction can be associated with inflammation or infection (cholangitis, pancreatitis, inflammatory bowel disease, cholelithiasis, liver neoplasia, cirrhosis, foreign bodies, abscesses, and parasites), although congenital diseases can create the same appearance.8-14
When all causes of obstruction have been ruled out, correlation with history, age, and clinical signs can support a diagnosis of congenital disease involving the biliary tree.
Congenital cystic changes of the biliary tree can affect any portion of the biliary tree, including the intra- and extrahepatic biliary ducts.2,5,9,15-18
The distribution of intrahepatic lesions can be examined with ultrasonography. The ultrasonographic appearance of the feline biliary tree has been well described in the literature.2,4,5,11,12,19-24
Although computed tomography (CT) and magnetic resonance imaging (MRI)25 can further characterize the location and aspect of the lesions, advanced imaging may not be recommended due to possible risks involving anaesthesia, age, and clinically unstable patients.
A 2-month-old DSH kitten was presented for delayed growth compared with the rest of the litter, polyphagia, and abdominal distension. Good general condition was reported.
Clinical examination revealed asymmetrical abdominal distension at the level of the right cranial abdomen, and generalized jaundice, with vital functions being within normal limits (heart rate 122 bpm; respiratory rate 32 rpm; temperature 38.9 °C).
A standard blood test was performed, haematology and biochemistry revealed moderate leucocytosis [WBC-25.96 (2.87-17.02x10^9/L)], mild neutrophilia [12.15 (2.30 - 10.29 x10^9/L)], lymphocytosis [9.53 (0.92 - 6.88 x10^9/L)], monocytosis [1.05 (0.05 - 0.67 x10^9/L)] and anisocytosis, creatinine [17 (53 - 141 µmol/L)], increased ALP [445 (14 - 192 U/L, values for young patients)], elevated GGT [144 (0 - 1 U/L)], bilirubin-total [ 81 (0 - 15 µmol/L)] and lipase [1,214 (40 - 500 U/L)]. The rest of the results were unremarkable. An abdominal ultrasound was, findings revealed moderate hepatomegaly with homogeneous echogenicity. Generalized distended and tortuous ducts (between 5-7 mm) were noted within all liver parenchyma (Figure 1-red arrow), with the gallbladder being identified separately, with normal architecture. In this case, the abdominal distension was mostly caused by the dilated intrahepatic ducts, mimicking hepatomegaly. The common biliary duct was normal, followed up to the level of the duodenum, duodenal papilla was not well identified owing to the amount of ingesta. Mildly thickened biliary duct walls were seen at the level of the central liver. No obvious cause of biliary obstruction was identified. Color Doppler flow was used to confirm the absence of blood flow at the level of distended ducts. Due to the size and age of the patient (400 g and 2 months of age), symptomatic treatment was started with ursodeoxycholic acid10 mg/kg PO q24h, liver supplements based on SAMe (S-Adenosyl-L-Methionine), Silybin, Turmeric extract (Curcumin) and amoxicillin and clavulanic acid, 15 mg/kg PO q12h. Abdominal distension improved after 5 days, but treatment was discontinued after 1 week.
Longitudinal (A) and transverse (B) image of the liver- Diffuse tubulo-saccular dilatation, mostly within the right liver, was noted, with secondary compression of the local hepatic veins and arteries, subsequently causing turbulent and high-velocity blood flow, assessed with PW Doppler. The GB was identified, but in a more lateral position, most likely due to compression and mass effect of the distended intrahepatic ducts.
On abdominal ultrasound, the liver was enlarged and distorted by numerous coalescing anechoic structures. In the absence of a Color flow Doppler signal and localization of the hepatic blood vessels, the structures were presumed to represent intrahepatic biliary ducts.
A follow-up ultrasound performed 4 weeks after the initial examination, revealed an increase in the distention of the intrahepatic bile ducts (> 7 mm) (Figure 2A-white arrow) with echogenic material formation within the ducts (Figure 2B-red arrow), thickening of the gallbladder wall (1.4 mm)10,27 and multilayer pattern (double rim aspect).
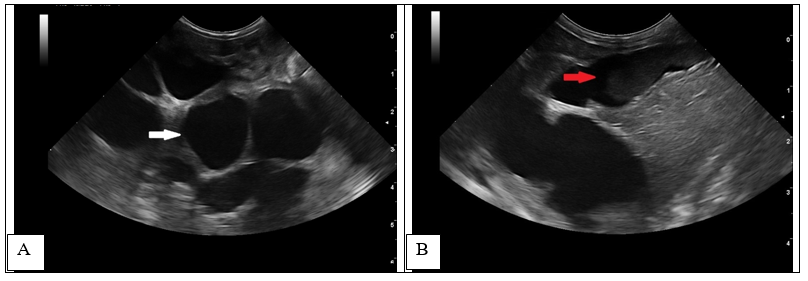
Figure 2 1-month follow-up ultrasound scan at the level of the liver (transverse and longitudinal images).
Increased dilatation of the intrahepatic bile ducts, with secondary compression of the local vasculature (A). Dependent echogenic debris is seen within the tubular bile ducts. Following the normal architecture of the bile ducts (localized in the proximity of the liver vasculature), the portal branches were difficult to visualize, causing the intraluminal portal vein sign16,27 (dilated ducts surrounding the portal vein) (Figure 3).
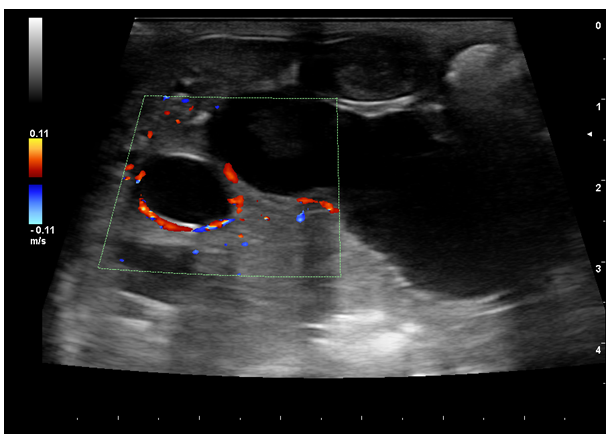
Figure 3 1-month follow-up ultrasound scan. Portal venous branches are partially surrounded by dilated bile - the intraluminal portal vein sign (red Doppler signal).
At the age of 4 months, a dual-phase (portal and delayed-venous) abdominal CT was performed to further describe the lesions and the extent of the process (SOMATON Scope CT VC40, 16-slice CT, Siemens, USA). A non-ionic iodinated contrast agent consisting of iohexol (Omnipaque™, GE Healthcare) was administered via an intravenous bolus. Post-contrast results of the examination were represented by hepatomegaly with large tubular structures, non-enhancing and hypoattenuating to the surrounding parenchyma, present throughout the liver (Figure 4A, C-red arrowhead). The tubular structures follow the approximate location of the biliary ducts with a branching pattern within the liver, close to the hepatic vasculature. (Figure 4A, B-orange arrowhead). There was difficulty encountered in identifying the gallbladder, as a result of numerous distended loops (Figure 1A-yellow arrows). A 9 mm, large, distended structure extending throughout the right limb of the pancreas to the region of the duodenal papilla was noted (Figure 4D-green arrows). The pancreatic duct was mildly distended, measuring less than 3 mm in diameter. The distended structures were filled with fluid attenuation content (10 – 20 HU) with a variably thickened wall (less than 4 mm). The hepatic changes had a marked mass effect, with right-sided displacement of the caudal vena cava noted due to the mass effect of hepatic changes. The mesenteric vasculature was relatively prominent, with many tortuous vessels present medial at the left kidney in the region of the left gastric vein and cranial mesenteric artery (Figure 4D-purple arrows). There was no evidence of a single well-defined congenital extrahepatic or intrahepatic shunt.
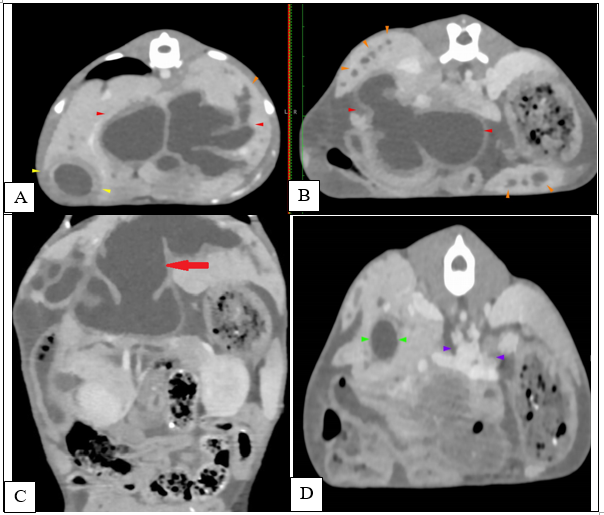
Figure 4 Transverse (A, B, D) and coronal (C) abdominal CT 0.75 mm slice image- Distended intrahepatic ducts causing a mass effect at the level of the cranial abdomen (red arrow) and pancreatic cyst (green arrow). A mild peritoneal effusion was noted, although this finding was unremarkable for the age of the patient.
Due to increased abdominal distension and echogenic sludge formation, treatment was re-initiated with the same medication in which clinical signs markedly improved (ursodeoxycholic acid10 mg/kg PO q24h, liver supplements based on SAMe (S-Adenosyl-L-Methionine), Silybin, Turmeric extract (Curcumin) and amoxicillin and clavulanic acid, 15 mg/kg PO q12h).
An ultrasonographic re-examination performed at 4 weeks showed a decrease in the intrahepatic bile duct size, however, transversal intraluminal septae at the level of the intrahepatic biliary tree were noted (Figure 5-white arrow). Portal flow velocity was recorded within normal limits, with regular flow (Figure 6).
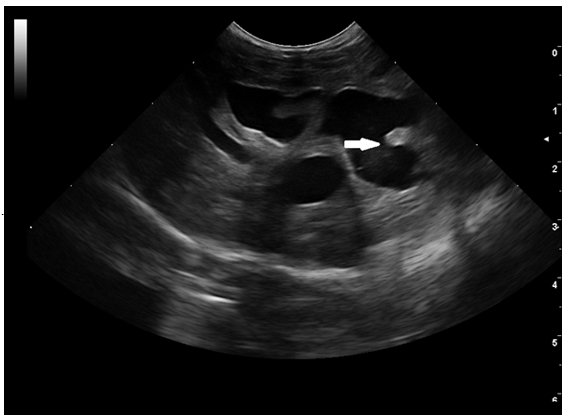
Figure 5 1-month follow-up ultrasound scan. Transverse image of the intrahepatic biliary tree. Septal formation (white arrow) was seen bridging the walls of the hyperechoic duct. Echogenic to hyperechoic bile was seen at the level of the left and central liver.
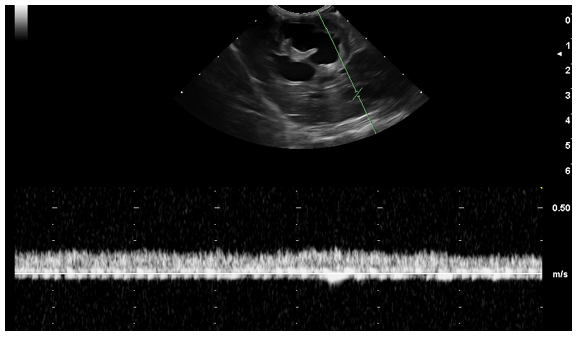
Figure 6 Transverse image of the portal flow velocity (20 cm/s). Normal portal vein wave was present.
The patient continued to improve, and the colour of the mucous membranes improved although continued to have an icteric appearance, abdominal distension decreased, and good clinical demeanour was reported.
From the same litter, a sibling was presented with a similar onset of clinical signs decreased appetite, generalised jaundice, vomiting, episodes of lethargy. A general blood test revealed non-regenerative microcytic anaemia, moderate elevation of the liver enzymes, and hyperbilirubinemia [134 (0 - 15 µmol/L)], GGT=[17 (0 - 1 U/L)], ALT=[1700 (10-125 U/L)].
Abdominal imaging has been performed (abdominal ultrasound and computer tomography). On the ultrasound, moderate distension of the intrahepatic and extrahepatic bile ducts has been recorded (2-6 mm). Multiple cystic-like components with hypoechogenic content disseminated through all the liver structures have been highlighted.
CT scans have been performed similarly to the previous case Moderate distension of the gallbladder with hypoattenuating fluid (10 HU) has been recorded. Variable distension of the extrahepatic bile ducts( 4.7 mm) and common bile duct (2-6.5 mm) with multiple tortuous tubular structures with hypoattenuating content and thick wall on the medial aspect of the gallbladder and ventral to the portal vein (Figure 7 - pink arrows). The exact termination of the duodenal papilla was not visible, but the distal aspect of the common bile duct measures normal (< 4mm) and there is an increased contrast uptake at the level of the papilla.
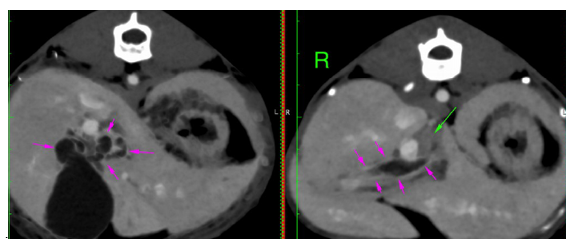
Figure 7 Distended extrahepatic bile ducts with tortuous trajectory. No obvious cause of obstruction has been seen on the CT scan. Moderate intraabdominal lymphadenopathy (hepatic - 1.1 cm, green arrows), jejunal and ileocaecocolic most severely, up to 8 mm, left/caudal colic 6-7 mm, have been seen with the rest of the abdominal and pelvic lymph nodes mildly enlarged.
Other findings have been represented by a diffusely enlarged liver with rounded edges, extending caudally from the coastal arch. Multiple small contrast-enhancing vessels with marked, but diffusely heterogeneous contrast uptake throughout the liver in the arterial phase have been seen (green circles) – Figure 8. The proportional size of the aorta, caudal vena cava, and portal vein at the level of the porta hepatica was relatively normal.
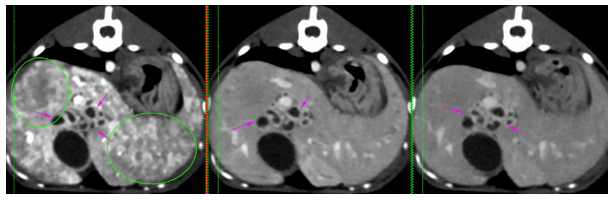
Figure 8 Multiple contrast-enhancing hepatic vessels. Distension on the bile ducts with marked thickened walls. No anomalous connections of the portal system have been seen.
As with the previous kitten, a small amount of peritoneal effusion in the caudoventral abdomen has been noted. Congenital anomaly and partial fusion of the coeliac and mesenteric arteries, mild generalized splenomegaly, and mild bilateral nephromegaly were also recorded.
Symptomatic treatment and fluid therapy have been started, similar to the kitten from the same litter, with liver enzymes improving after 4 weeks. One month after starting the treatment, the second kitten started to gain weight compared with the sibling (3.7 kg, from 3.1 kg). 1-month post-treatment, blood results revealed a decrease in the liver enzymes (ALT 102 U/L, ALK=62 U/L). Treatment has been continued for another month; clinical signs have improved. As in the previous case, ultrasound examination follow-up highlighted transverse septae and echogenic to hyperechoic bile. The intraluminal portal vein sign (dilated ducts surrounding the portal vein) was noted as well during the ultrasound examinations and may represent a sign usually associated with bile duct ectasia, congenital or acquired.
Compared with the sibling, no cysts have been seen on the imaging examination, and a difference in the size of the intra- and extrahepatic bile ducts has been noted, in the second case being smaller, possibly related to the later onset of clinical signs. Although the changes in the biliary tree were reduced compared with the siblings, a genetic transmission can be suspected, as previously mentioned in the literature.11,13
Treatment has been continued, and current clinical behaviour is normal, although occasional reoccurrence of the clinical signs has been reported.
Congenital intrahepatic bile duct ectasia is a rare hereditary disorder of the biliary tree in dogs and cats, with a limited number of published reports documenting this pathology.19 Complete ultrasound examination may facilitate differentiating from other conditions and aid diagnosis, especially when cystic lesions are recorded in other abdominal organs (pancreas, kidneys).3,12,19 Clinical signs associated with the disease are represented by acute abdominal pain, lethargy, vomiting and diarrhoea, jaundice, and abdominal distension in cases of hepatomegaly or ascites due to portal hypertension.10,12,22 Similarly, a delay in growth may be seen in some patients when systemic shunts or arteriovenous fistulisations coexist. In human patients, hematemesis and melena secondary to bleeding varices are well described, although in veterinary medicine, these clinical signs are uncommon, possibly related to the rarity of the disease or the difficulty encountered in obtaining a definitive diagnosis. Congenital intrahepatic bile ducts ectasia is more likely to occur in the presence of other diseases, such as autosomal recessive polycystic kidney disease, cholangitis, gallstones, biliary abscess, sepsis, liver cirrhosis, kidney failure, and cholangiocarcinoma.4,5,18,22,25
Further characterized by additional imaging modalities, the disease may be diffuse, lobar, or segmental. Dilatation of the intrahepatic bile ducts is most frequently tubulo-saccular rather than fusiform, a feature that might aid diagnosis, also correlated with age and the absence of extrahepatic biliary obstruction.18,22 Identification of the GB and the connection of the extrahepatic bile ducts to the CBD are paramount in diagnosing these anomalies.19
In the present cases, advancement of the pathological process, intraductal bridging, echogenic septae formed by bile accumulation, gallbladder wall oedema, and thickening of the intrahepatic duct walls were seen during the ultrasonographic examination. The intraluminal portal vein sign16 (dilated ducts surrounding the portal vein) was noted during the ultrasound examinations.
Further imaging modalities including CT examination, may show multiple hypoattenuating rounded areas that are inseparable from the dilated intrahepatic bile ducts, in this instance, the central dot sign,22,26 associated with Caroli disease in human medicine27 (enhancing dots within the dilated intrahepatic bile ducts, representing portal radicles) was not visualized, possibly related with the small size of the patient and nonuniform intravascular contrast solution. Differential diagnoses include other diseases that can have the same imaging appearance polycystic liver disease, choledochal and peribiliary cysts, cholangitis, and biliary obstruction.1,2,15,17,21,27,29-33 Imaging modalities can support the diagnosis, especially when the intrahepatic bile ducts are seen surrounding the portal vasculature, having a tubular structure that follows up to the level of the gallbladder.28
Although the size of the intrahepatic bile ducts was considerably decreased after the administration of symptomatic medication, intraluminal transverse septae and hyperechoic bile duct walls were observed at the level of the biliary tree, presuming that septal changes can be associated with chronicity of the pathological process.
Even though portal flow was recorded as turbulent when first diagnosed, velocity became normal, a possible relation to the adaptive physiological mechanisms.
Caroli's disease, or congenital intrahepatic bile duct ectasia, is a rare disorder in small animals. Differential diagnosis needs to be made primarily with single isolated choledochal cysts, polycystic liver disease, and biliary obstruction. Even though a biopsy is required to confirm the diagnosis, in cases when this cannot be achieved (clinically unstable patients or if the owners declined), the age of the patient and presentation can be supportive for a description of congenital malformations. Also, imaging findings like isolated cysts in other organs can help in the diagnosis. After the previous presentation of the patient, a sibling from the same litter was presented with the same clinical signs, imaging findings being similar, exception being made of the pancreatic cyst. This can prove the genetic relation in the transmission and the evolution of bile duct ectasia in cats. To the author's knowledge, this is the first case report description of a congenital intrahepatic bile duct ectasia coevolving with a pancreatic cyst in cats, with biliary tree changes seen in siblings from the same litter. In the presented cases, the intraluminal portal vein sign (dilated ducts surrounding the portal vein) was noted during the initial ultrasound examinations and may assist with the diagnosis.
Furthermore, as the disease advances, transverse septae at the level of the bile ducts, gallbladder rim, and thickening of the biliary tree have been noted. Even if the initial PW Doppler recorded turbulent flow, follow-up examinations showed normal velocity of the portal vasculature. This may be in relation to treatment response or adaptive physiological mechanisms. Imaging modalities can be used to describe and monitor Caroli's disease, biliary tree changes, and cysts at the level of abdominal organs, but also as a response to symptomatic treatment.
Even if advanced imaging methods (CT) provide a better description of the pathological process, abdominal ultrasound is a more accessible method for evaluating the bile duct in cats and can be used to monitor the evolution of the disease dynamically.
No funds have been received for this work.
Some results from the article have been published in another journal, the present one being more comprehensive and descriptive.
The authors declare they have no conflicts of interest.
No experimental procedures were performed, and thus no ethical committee approval was required.

©2025 Cristi. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.