International Journal of
eISSN: 2573-2889


Research Article Volume 8 Issue 1
Department of Chemistry, University of Puerto Rico at Mayagüez, USA
Correspondence: Elsie I Parés Matos, Department of Chemistry, University of Puerto Rico at Mayagüez, Mayagüez, Puerto Rico, 00680, USA, Tel +1 7878324040
Received: May 31, 2025 | Published: June 9, 2025
Citation: Fajardo MMC, Matos EIP. Determining the role of FAP1 in S. cerevisiae, cultivated in Nitrogen-limited media. Int J Mol Biol Open Access. 2025;8(1):34-43. DOI: 10.15406/ijmboa.2025.08.00195
FAP1 is a cytoplasmic protein found in Saccharomyces cerevisiae that can bind Fpr1p for conferring Rapamycin’s resistance to yeast. Rapamycin is lethal to yeast because it can also bind Fpr1p, and this complex interferes negatively with the TORC1 function, which is responsible for nutrient-regulated gene expression. Currently, the FAP1’s function remains poorly understood, but it has been suggested that under nutrient-deficient conditions, the fap1 gene is over-expressed and becomes essential for yeast to survive. In this study, the expression levels of the fap1 gene were monitored when yeast cells were exposed to Nitrogen-limited media for 0 h, 3 h, 6 h and 9 h. After the harvesting yeast cells, total RNA was extracted and treated with Reverse Transcriptase to generate complementary DNA (cDNA), after which concentrations ranging from 1939 ng/µL (0 h), 1608 ng/µL (3 h), 1845 ng/µL (6 h) and 1521 ng/µL (9 h) were obtained. Real-time (quantitative) PCR experiments were performed using these cDNA samples and those obtained after yeast cells were grown in YPD (control media) at 0 h. This experimental method was used to validate the amplification efficiency of the fap1 gene relative to the endogenous fba1 gene, by establishing the conditions necessary for acquiring experimental cycle threshold (CT) values. The obtained results suggest that the expression levels of the fap1 gene increase, under different Nitrogen-limited media, as time progresses.
Keywords: FAP1, gene expression, nitrogen limited media, qPCR, Saccharomyces cerevisiae
FAP1, FKBP12 Associated Protein 1; Fpr1p, FK506-sensitive Proline Rotamase 1; FKBP12, FK506 Binding Protein 12 (human homolog of Fpr1p) fap1; FKBP12, Associated Protein 1 gene (yeast); TOR, Target of Rapamycin; TORC, Target of Rapamycin Complex; cDNA, complementary DNA; SSY1, Sulfonylurea Sensitive on YPD; NLM, Nitrogen-limited media; YPD, Yeast Extract Peptone and Dextrose (complete media); YNB, Yeast Nitrogen Base
Saccharomyces cerevisiae (S. cerevisiae) is a powerful yeast cell that possesses a nuclear genomic DNA, organized into 16 chromosomes, where about 5570 potential protein-encoding genes have been found.1 Its predominant mode of vegetative reproduction is based on budding.2 S. cerevisiae has been widely used for studies on nutrient-sensing and signaling mechanisms. Some of the regulatory mechanisms developed in yeast involve arginine activation and proline utilization pathways by their substrates, regulation of genes involved in the general control of amino acid biosynthesis and the transcriptional regulation of certain amino acid permeases by the SSY1 pathway for sensing amino acids in the medium.3 Nitrogen regulation is another mechanism used to prevent or reduce differences based on synthetic capacity. For example, in response to lack or poor-quality nitrogen sources in the growth medium, yeast cells increase the expression of enzymes responsible for glutamate and glutamine biosynthesis, thus increasing the activity of those permeases involved in the uptake of amino acids for their use as a nitrogen source. According to Magasanik’s group, Glutamine is preferred as a nitrogen source because it can support faster growth than all non-preferred sources of nitrogen.3 However, differences in growth rate supported by very different nitrogen sources are often surprisingly small, therefore, it is difficult to use growth rate to make clear distinctions between the qualities of different nitrogen sources. These observations allow researchers to establish new concepts and knowledge into nutrient-controlled cellular regulation, thus leading to the activation or deactivation of different mechanisms that allow yeast cells to survive.4 In the year 2000, Kunz’s research group reported that a yeast protein called FAP1 confers Rapamycin resistance by competing for the Fpr1p binding site.5 They also reported that FAP1 translocate to the nucleus, as shown in Figure 1, where it can function as a possible transcription regulator. Although the proposed mechanism by which FAP1 gives yeast cells resistance to Rapamycin is still unclear, it seems that yeast cells can undergo a starvation process, approximating those observed under Nitrogen-limited conditions. Thus, it is possible to consider FAP1 as a direct link between nutrient availability and yeast survival. Additionally, FAP1 harbors a putative DNA-binding motif similar to several mammalian transcription factor regulators, including the human homolog NFX-1.5 Matsuo and Inada have also explained that FAP1 might be involved in the degradation pathway for the non-functional ribosome.6 Thus, the lack of FAP1 expression could be detrimental to yeast since the formation of the Rapamycin:Fpr1p complex has shown to be linked to the inactivation of TORC1 and, as a consequence, yeast cells are induced to suffer from nutrient deprivation.7,8 However, if overexpression of the fap1 gene occurs, in a nutrient-limited medium, then a direct link between nutrient availability and FAP1 overexpression could exist. For that reason, we propose that FAP1 is one of the yeast proteins that might help yeast cells to survive under stress conditions under nutrient deficiency. Thus, the overexpression of the fap1 gene could be used as a biomarker when yeast cells are exposed to Nitrogen-limited media (as one of the stress conditions to be employed).
Yeast cultured in yeast extract-peptone-dextrose (YPD) broth
A small sample of a frozen stock of the yeast strain CFY7-PJ69-6A was grown on a YPD-agar plate and incubated at 32°C for 3-4 days.9 Subsequently, a single yeast colony was inoculated into 10.0 mL of YPD broth with 100 µL (34 mg/mL) Chloramphenicol and incubated with constant agitation (150 rpm) at 30°C.10 The cultured yeast was scaled up and grown until an OD600 0.7-0.9 was reached. Once the OD600 0.7-0.9 was reached, cultured yeast cells were centrifuged at 4000 rpm for 10 min at 30°C to obtain the yeast cell pellet. Figure 2 shows the steps required while culturing yeast cells.
Induction of cultured yeast cells in nitrogen-limited media (NLM)
Yeast cell pellets were resuspended in a medium that contained 0.34 g/mL of Yeast Nitrogen Base (YNB) without amino acids and ammonium sulfate (HiMedia Laboratories, Ref M151-100G), supplemented with 0.006, 0.125 g or 0.25 g of ammonium sulfate.11 Resuspended yeast cells were further incubated with constant agitation (150 rpm) at 30°C for 0 h, 3 h, 6 h and 9 h. The induction was stopped when an aliquot of 10 mL of yeast cells was centrifuged at 4000 rpm for 10 min at 4°C. Yeast pellets were immediately frozen at -20°C until the next step. Figure 3 shows the steps required for the induction of cultured yeast cells.
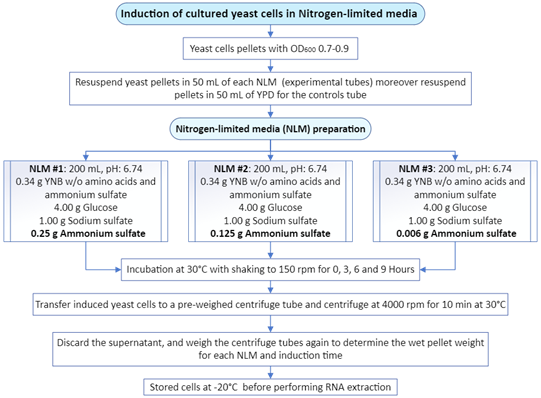
Figure 3 Induction conditions for the exposure of cultured yeast cells to a Nitrogen-limited media (NLM) that contains different amounts of Ammonium Sulfate.
Conversion of FAP1 mRNA into FAP1 cDNA
Total RNA was extracted after a lysis reaction of each yeast pellet with the PureLink RNA Mini Kit Protocol (ThermoFisher Scientific, catalog number 12183018A). For the NLM1 samples and controls, the extraction was performed using mechanical disruption, involving the addition of 0.5 mL of Lysis Buffer, and following the steps according to Figure 5.3. Samples and controls from NLM2 and NLM3 were extracted using enzymatic disruption with Zymolyase (ZYMO RESEARCH, E1004/E1005) employing 10 µL of Zymolyase digestion buffer for samples below 0.1 g of yeast frozen pellet weight, while 20 µL of Zymolyase was used for samples over 0.1 g. Figure 4 shows the general steps required for total RNA extraction. After extracting the total RNA, the purification process was conducted using a series of washes and silica-membrane columns, following the steps outlined in Figure 5. Subsequently, the total RNA was transformed into cDNA by a reverse transcription (RT) reaction with the Applied Biosystems High-Capacity cDNA Reverse Transcription Kit Protocol (Fisher Scientific, catalog number 43-688-14). The conditions required for every amplification step using a thermal cycler machine are shown in Table 1. The concentration of each synthesized cDNA was determined using the Thermo Scientific™ NanoDrop™ Microvolume UV-Vis Spectrophotometer (Serie AZY2333437). Figure 6 shows the steps required for the preparation of cDNA.
|
Conditions |
Step 1 |
Step 2 |
Step 3 |
Step 4 |
|
Temperature (°C) |
25 |
37 |
85 |
4 |
|
Time |
10 min |
120 min |
5 min |
Hold |
Table 1 Thermal cycler parameters for the synthesis of cDNA
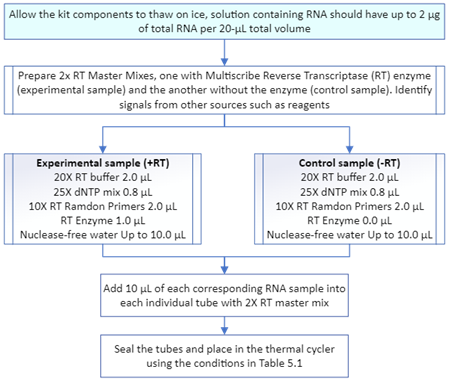
Figure 6 Synthesis of cDNA from total RNA using High-Capacity cDNA Reverse Transcription Kit Protocol.
qPCR experiments
Validation protocol
Validation was carried out to demonstrate that the experimental gene (fap1) and the endogenous gene (fba1) have similar or comparable amplification efficiency. A calibration curve was constructed for each gene in which both must have a slope around -3.32 ± 10%, confirming that the reaction efficiency falls within the acceptable range of 90-110%.12 Efficiency was calculated using Equation 1 which involves the slope of the calibration curve.13
Equation 1 Efficiency = 10-(1/slope)-1
The quantitation of gene expression was run using Real-Time Quantitative PCR. A serial dilution of the calibrator sample was necessary to generate the calibration curve.14 This process prevents abrupt signals in qPCR resulting from high concentrations of cDNA and minimizes signals from any potential contamination. After obtaining the calibration curves, a plot of Delta CT versus cDNA quantity Log was generated to determine the slope between CT of fap1 and fba1. If the absolute value of the slope is close to zero, it indicates that the efficiencies of the target and reference genes are similar across all input concentrations.12 A slope <0.1 is generally considered acceptable when employing the ∆∆CT method. The cDNA concentration was determined using the Thermo Scientific™ NanoDrop™ Microvolume UV-Vis Spectrophotometer (Serial Number AZY2333437) to calculate the dilutions. Each sample was diluted to ensure an equal concentration of cDNA (100 ng/µL) and was analyzed in triplicate. The parameters for the thermal cycler were determined based on the primers and master mix, as detailed in Table 2. The TaqMan Universal PCR Master Mix protocol (Fisher Scientific, catalog number 43-044-37) stipulates that for qPCR, each well should contain half of the total volume of TaqMan™ Fast Advanced Master Mix.
|
|
Holding stage |
Holding stage |
Cycling stage (n=40) |
|
|
Step 1 |
Step 2 |
Step 1 |
Step 2 |
|
|
Temperature (°C) |
50 |
95 |
95 |
60 |
|
Time |
02:00 min |
00:20 min |
00:01 min |
00:20 min |
Table 2 Conditions for qPCR using the TaqMan® Universal PCR Master Mix
Quantification of FAP1 cDNA expression
Once cDNA dilutions for each sample were prepared, the experiment was conducted using the comparative CT method (∆∆CT) with the Applied Biosystems StepOne™ Real-Time PCR System (Serial Number 2710005037). The primers designed for these experiments are listed in Table 3. Primers were synthesized and acquired through Custom Taqman® Gene Expression assays (Applied Biosystems, APEPXNV, FBA1-VIC PN 44485083) and (Applied Biosystems, APDJ33X, FAP1-FAM PN 43313483). Each custom assay consists of a mixture of forward and reverse primers, along with a FAM™ or VIC™ dye-labeled TaqMan® MGB probe. A total reaction volume of 20 µL was used per well, with negative controls containing only RNase-free water. Using 1 µL of fba1 and 1 µL of fap1 in a single qPCR reaction was employed to detect the two targeted gene sequences simultaneously. The setup for the 48-well plate is shown in Table 4. These experimental methods were created and executed using StepOne Software V2.3 under the names validationfap1fba1.eds and the deltactNLMx3.eds, to compare the expression levels between fba1 and fap1.
|
fap1 gene |
Nucleotide sequence |
Length (nt) |
Melting temperature (°C) |
GC% content |
|
Forward Primer |
GGTAAGTCGTCGAAGGATGAAA |
22 |
62 |
45.5 |
|
Probe |
TAGGCGTGTTTGCCTGTGCAGATA |
24 |
68 |
50 |
|
Reverse Primer |
GAATGAATGCTTGCGACATGAG |
22 |
62 |
45.5 |
|
fba1 gene |
Nucleotide Sequence |
Length (nt) |
Melting Temperature (°C) |
GC% Content |
|
Forward Primer |
AGATCCATTGCTCCAGCTTAC |
21 |
62 |
47.6 |
|
Probe |
ACACTCTGACCACTGTGCCAAGAA |
24 |
68 |
50 |
|
Reverse Primer |
GCTTCATCAGCTTCCAACATAC |
22 |
62 |
45.5 |
Table 3 Primers designed and used for the fap1 and fab1 genes based on nucleotide length (nt), melting temperature (°C), and Guanine/Cytosine content (GC%)
|
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
|
A |
Negative Control |
M reference |
NLM (X h) FAP1 FBA1 |
NLM (X h) FAP1 FBA1 |
NLM (X h) FAP1 FBA1 |
YPD (X h) FAP1 FBA1 |
YPD (X h) FAP1 FBA1 |
YPD (X h) FAP1 FBA1 |
|
B |
NLM (X h) FAP1 FBA1 |
NLM (X h) FAP1 FBA1 |
NLM (X h) FAP1 FBA1 |
YPD (X h) FAP1 FBA1 |
YPD (X h) FAP1 FBA1 |
YPD (X h) FAP1 FBA1 |
NLM (X h) FAP1 FBA1 |
NLM (X h) FAP1 FBA1 |
Table 4 StepOne™ 48 well set up for Comparative C_T (ΔΔCt) analysis for X hour of cell harvesting time. The experiments were run in triplicate. Only 16/48 samples are shown
The relative quantification technique was employed using the comparative CT method for data analysis, as it applies to most gene expression studies. This technique involves assaying the expression levels of a gene of interest for up- or down-regulation in both a calibrator (normal) sample and one or more experimental samples.14 Precise copy number determination is not necessary with this technique, which instead focuses on fold change compared to the calibrator sample.15
Comparative quantification algorithms - ∆∆Ct
The comparative CT method employs the Ct difference between the target gene in the test and calibrator samples, normalized to the reference gene Ct values and adjusted for minute variations in amplification efficiency. The average ΔCt between the normalizer and target gene can be obtained for each dilution according to equations 1 to 4. The value itself is not important; it is the consistency of that value across each dilution that matters.16 The calibrator sample undergoes the same purification procedure, is involved in the same reactions, and has similar complexity to the experimental samples. Equations 2 to 5 were used to determine ∆∆Ct.
Equation 2 Ct fap1sample – Ct fba1sample = ΔCt sample
Equation 3 Ct fap1calibrator – Ct fba1calibrator = ΔCt calibrator
Equation 4 ΔCt sample –ΔCt calibrator = ΔΔCt
Equation 5 Fold difference = 2–ΔΔCt
Yeast cultured in yeast extract-peptone-dextrose (YPD) broth
Initially, cell cultures were cultivated to obtain yeast pellets (exponential phase), which were subsequently subjected to induction for up to 9 hours in three nitrogen-limited media with varying ammonium sulfate concentrations. Table 5 shows the average OD600 and pellet weight obtained from three tubes for each NLM and their corresponding controls used in cell induction.
|
Assay |
OD600 |
YPD cultures (control) |
NLM cultures (experimental) |
|
Pellets weight (g) for induced yeast cells |
|||
|
NLM1 |
0.79 ± 0.07 |
0.09 ± 0.03 |
0.08 ± 0.02 |
|
NLM2 |
0.88 ± 0.04 |
0.11 ± 0.02 |
0.11 ± 0.01 |
|
NLM3 |
0.87 ± 0.04 |
0.08 ± 0.01 |
0.11 ± 0.01 |
Table 5 Average OD600 and pellet weight used in cell inductions (N=3)
Induction of cultured yeast cells in nitrogen-limited media (NLM)
According to the described methodology, this section presents the obtained results, focusing on the weight of the induced yeast cell pellets and their corresponding controls. The induction was conducted at 0 h, 3 h, 6 h and 9 h, by resuspending the yeast pellets from the previous step in three nitrogen-limited media (NLM1, NLM2, NLM3), each containing varying amounts of ammonium sulfate: 0.250 g, 0.125 g, and 0.006 g, respectively. Corresponding controls were also resuspended in YPD media and collected simultaneously. The average weights obtained from three assays conducted for each NLM are summarized in Table 6.
|
Time (hours) |
YPD cultures (control) |
NLM cultures (experimental) |
||
|
Pellets weight (g) for replicates with NLM1 |
||||
|
|
Average |
SD |
Average |
SD |
|
0 |
0.07 |
0.05 |
0.07 |
0.04 |
|
3 |
0.13 |
0.04 |
0.08 |
0.04 |
|
6 |
0.19 |
0.02 |
0.08 |
0.06 |
|
9 |
0.25 |
0.01 |
0.08 |
0.01 |
|
|
Pellets weight (g) for replicates with NLM2 |
|||
|
|
Average |
SD |
Average |
SD |
|
0 |
0.08 |
0.03 |
0.03 |
0.03 |
|
3 |
0.07 |
0.02 |
0.04 |
0.02 |
|
6 |
0.11 |
0.02 |
0.04 |
0.02 |
|
9 |
0.18 |
0.02 |
0.03 |
0.01 |
|
|
Pellets weight (g) for replicates with NLM3 |
|||
|
|
Average |
SD |
Average |
SD |
|
0 |
0.07 |
0.03 |
0.03 |
0.01 |
|
3 |
0.13 |
0.02 |
0.04 |
0.01 |
|
6 |
0.14 |
0.02 |
0.03 |
0.01 |
|
9 |
0.19 |
0.02 |
0.03 |
0.01 |
Table 6 Average weight for pellets obtained from induced yeast cells and their corresponding controls (N=3)
Conversion of FAP1 mRNA into FAP1 cDNA
After cell induction, RNA extraction was essential for cDNA synthesis. Total RNA extraction was conducted for each sample, accordingly, followed by purification to reach a total volume of 200 µL per sample. The results suggest no significant difference in total RNA concentration between control and NLM1 at 0 h. However, a significant difference between controls and NLM1 for 3 h, 6 h and 9 h was notable as observed in Figure 7A. On the other hand, no significant difference was observed for controls and NLM2 at 0 h, 3 h, 6 h and 9 h. For NLM3, there is no significant difference between controls and NLM3 at 0 h and 9 h. Moreover, a significant difference between controls and NLM3 was only observed for 3 h and 6 h. Results for the averaged total RNA concentration for NLM2 and NLM3 are shown in Figure 7B and Figure 7C, respectively. The total RNA concentration, for the controls at 3 h and 6 h, was higher than for the induced cells because a large amount of starting material was used, and the total RNA yield depended on the initial sample size. Consequently, once total RNA was extracted and their concentrations were determined, samples were diluted to a final volume of 30 µL if the concentration exceeded 0.2 µg/µL, ensuring compatibility with the reverse transcription kit working range. Overall, the results suggest no significant difference in cDNA concentration between the controls and the induced cells in NLM (Figure 8). For this reason, the total RNA was finally diluted to the same concentration, and 10 µL of each sample was used for the PCR reaction with reverse transcriptase.
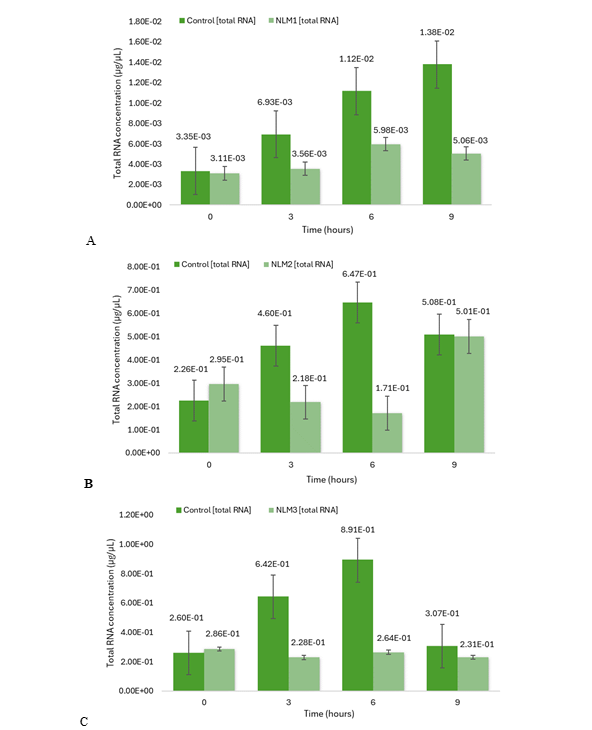
Figure 7 Total RNA concentration determined by QUBIT 3.0. Cells were incubated in NLM1 (A), NLM2 (B) and NLM3 (C), and in YPD medium for 0 h*, 3 h**, 6 h*** and 9 h**** before RNA was extracted. N = 3 (triplicates). Mean ± SEM is plotted. (A) *p < 0.799; **p < 0.007; ***p < 0.067; ****p < 0.050; (B) *p < 0.334; **p < 0.123; ***p < 0.140; ****p < 0.688; (C) *p < 0.593; **p < 0.00074; ***p < 0.052; ****p < 0.525. p values were determined by a two-tailed t-test.
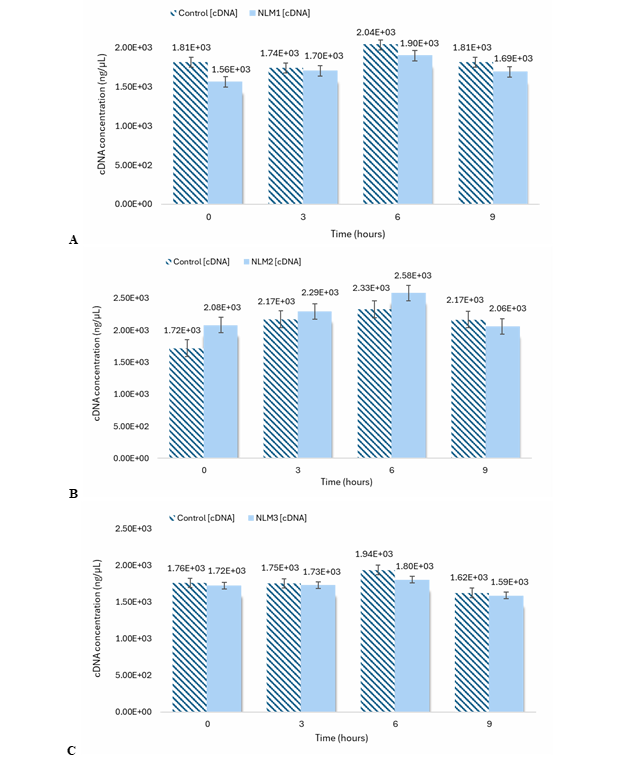
Figure 8 cDNA concentration determined by NanoDrop™ Microvolume UV-Vis Spectrophotometer. Cells were incubated in NLM1 (A), NLM2 (B) and NLM3 (C), and in YPD medium for 0 h*, 3 h**, 6 h*** and 9 h****. N = 3 (triplicates). Mean ± SEM is plotted. (A) *p < 0.402; **p < 0.864; ***p < 0.256; ****p < 0.524; (B) *p < 0.225; **p < 0.424; ***p < 0.061; ****p < 0.780; (C) *p < 0.788; **p < 0.796; ***p < 0.246; ****p < 0.777. p values were determined by a two-tailed t-test.
qPCR experiments
Validation protocol
The calibrator sample (also known as calibration sample) was taken from one of the controls for the 0-hour time point. In studies examining different time conditions, it is highly recommended for the calibrator sample to represent either the normal condition or a sample from a healthy individual. This is because reference genes should exhibit constitutive expression in cells regardless of environmental changes. However, since there's no universal reference gene whose expression remains unaffected by experimental conditions, it is necessary to validate the amplification of the experimental gene against a control gene, as in this case, fba1 was selected as the control gene because appeared to be one of the most reliable.17 The calibrator sample was prepared from a cDNA sample with an average concentration of 1365.2 ng/µL, which was then diluted to 100 ng/µL. To ensure the quality of the DNA sample, all absorbance readings were measured using a Nanodrop spectrophotometer, yielding a sample concentration of 102 ng/µL, and a purity of (A260/280 =) 1.89 and (A260/230 =) 2.37. The absorbance ratios 260/280 and 260/230 are indicators of quality and DNA usability, respectively. An absorbance 260/280 ratio of ∼1.8 is generally accepted as pure DNA. If the absorbance 260/280 ratio is ≤1.6, it may indicate the presence of proteins, phenol, or other contaminants that absorb strongly at 280 nm. However, the presence of RNA can lead to the ratio increasing. The absorbance 260/230 ratio is widely used as a second statement of DNA purity, where values over 2.0 and 2.2 are accepted. If the ratio is appreciably lower than expected, it may indicate the presence of contaminants that absorb at 230 nm such as proteins, guanidine HCl (used for DNA isolations), EDTA, carbohydrates, lipids, salts, or phenol.18
Subsequently, a serial dilution (starting from 100 ng/µL) was performed for our validation protocol. However, it was observed that at concentrations below 0.39 ng/µL, the Ct values became very high to the point that it could result in an indeterminate value. This is because the qPCR reaction involves 40 cycles, where the Ct value indicates the cycle from which the fluorescence signal begins to be detected. Additionally, the standard deviation (SD) shows an increase in the error with higher Ct values. Even though qPCR is limited by the number of run cycles, it is common practice to set an experimental Ct cutoff before the final cycle. Consequently, Ct value determination is unlikely to be a major source of error when calculating normalized expression.19 Thus, a Ct value of up to 36 can be obtained for a concentration of 0.39 ng/µL in this research work. The amplification efficiency was determined using Equation 1 with the slope of the line obtained in Figure 9, resulting in 95.43% for fap1 and 91.92% for fba1. After the calibration curve, a plot of ΔCT versus Log cDNA dilution was generated as shown in Figure 10. A slope of 0.0955 was obtained which is less than 0.1 to validate the use of the ∆∆CT method. The experimental results demonstrated that (for validation) fap1 and fba1 expression genes have similar amplification efficiencies within the concentration range of (100-0.39) ng/uL.
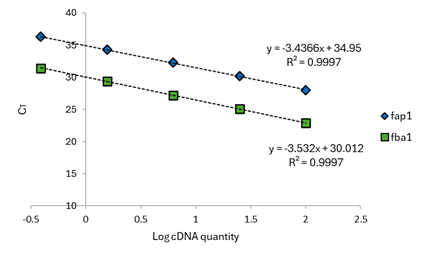
Figure 9 Relative standard curves for fap1 and fba1. A serial dilution of the calibrator sample (100 ng/µL) was necessary for generate the calibration curves. The serial dilutions factor was 1/4 and the standards were amplified by qPCR using gene-specific primers. N = 3 (triplicates). The most concentrated sample contained cDNA derived from 10 ng of total RNA.
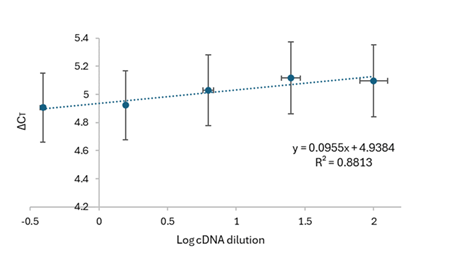
Figure 10 Validation efficiencies of fap1 against fba1 as a control gene. The standard curves for fap1 and fba1 during validation is shown. N = 3 (triplicates). The ∆CT (using Equation 2) was calculated for each cDNA dilution.
Quantification of FAP1 cDNA expression
The Ct, which stands for threshold cycle, denotes the endpoint of real-time PCR. The primers employed in the reaction were labeled with fluorophores: FAM for fap1 and VIC for fba1. This double labeling enables duplex reactions, as FAM emits fluorescence at 520 nm and VIC at 550 nm.20 Relative quantification was employed to assess the results obtained through real-time PCR, utilizing the ∆∆CT method for data analysis.
Data analysis
All cDNA samples were diluted to 100 ng/µL, mirroring the calibrator sample. Ct values were determined for each sample (in triplicate) and their corresponding control gene. Table 7-9 summarize the Ct values for the first analysis done using NLM1, NLM2, and NLM3, respectively, as induction media. These samples are used as examples to show the analysis performed. Their ΔCt values were calculated using Equation 2 to Equation 5. Each table displays the relationship between the average of the triplicate for each gene per sample, including the reference sample. The value of the relative quantity (RQ) or fold change for the reference sample is 1. In general, the fap1 gene shows RQ values with a fold increase when its data is analyzed from t=0 h to t=3 h, from t=3 h to t=6 h and from t=6 h to t=9 h. This trend is observed at all NLM conditions and suggests that the expression of the fap1 gene is independent of ammonium sulfate concentration. This assumption is also supported by the analysis of variance (ANOVA), a method commonly used for multiple comparisons. This method uses a single test to determine whether there is or not a difference among the population means in response to different factors (Skoog and West, 2014). However, while performing a two-factor ANOVA, no significant effect for the factor corresponding to the media variability was observed. The F-value was 3.0555, with a corresponding p-value of 0.1216. As the p-value exceeds 0.05, there is insufficient evidence to reject the null hypothesis (H0: μ1 = μ2 = μI), suggesting no significant differences among the three nitrogen-limited media (that is, the ammonium sulfate concentration). In contrast, the factor corresponding to time (0 h, 3 h, 6 h and 9 h) yielded an F-value of 26.125 and a p-value of 0.000765. With the p-value falling below 0.05, there is significant evidence to reject the null hypothesis, indicating differences among sampling times. Therefore, it can be concluded that the "Time" factor has a substantial influence on cDNA concentration.
|
Sample name |
Target |
Cт Mean |
ΔCт Mean |
ΔCт SE |
ΔΔCт |
2^(-) RQ |
RQ Min |
RQ Max |
|
reference sample |
fap1 |
27.965 |
5.191 |
0 |
1 |
|
|
|
|
reference sample |
fba1 |
22.775 |
|
|
||||
|
NLM1, t=0 h |
fap1 |
30.490 |
4.663 |
0.172 |
-0.528 |
1.442 |
0.863 |
2.408 |
|
NLM1, t=0 h |
fba1 |
25.827 |
|
|
||||
|
Control, YPD, t=0 h |
fap1 |
32.700 |
5.197 |
0.234 |
0.007 |
0.995 |
0.495 |
2.002 |
|
Control, YPD, t=0 h |
fba1 |
27.502 |
|
|
||||
|
NLM1, t=3 h |
fap1 |
29.794 |
3.302 |
0.015 |
-1.889 |
3.704 |
3.537 |
3.879 |
|
NLM1, t=3 h |
fba1 |
26.492 |
|
|
||||
|
Control, YPD, t=3 h |
fap1 |
29.717 |
5.679 |
0.063 |
0.488 |
0.713 |
0.591 |
0.860 |
|
Control, YPD, t=3 h |
fba1 |
24.038 |
|
|
||||
|
NLM1, t=6 h |
fap1 |
29.263 |
1.666 |
0.072 |
-3.525 |
11.509 |
9.296 |
14.249 |
|
NLM1, t=6 h |
fba1 |
27.597 |
|
|
||||
|
Control, YPD, t=6h |
fap1 |
28.181 |
3.834 |
0.050 |
-1.357 |
2.561 |
2.208 |
2.970 |
|
Control, YPD, t=6h |
fba1 |
24.347 |
|
|
||||
|
NLM1, t=9 h |
fap1 |
28.962 |
1.660 |
0.021 |
-3.531 |
11.561 |
9.588 |
13.940 |
|
NLM1, t=9 h |
fba1 |
27.346 |
|
|
||||
|
Control, YPD, t=9 h |
fap1 |
26.048 |
2.120 |
0.060 |
-3.071 |
8.405 |
7.034 |
10.043 |
|
Control, YPD, t=9 h |
fba1 |
23.928 |
|
|
Table 7 Results for the first analysis for Comparative C_T (〖∆∆C〗_T) after cell harvesting at 0 h, 3 h, 6 h and 9 h using NLM1 (0.250 g/mL (NH4)2SO4). The experiments were run in triplicate
|
Sample name |
Target |
Cт mean |
ΔCт mean |
ΔCт SE |
ΔΔCт |
2^(-) RQ |
RQ min |
RQ max |
|
reference sample |
fap1 |
26.900 |
5.013 |
0.073 |
0.000 |
1.000 |
0.524 |
1.907 |
|
reference sample |
fba1 |
21.887 |
|
|
|
|||
|
NLM2, t=0 h |
fap1 |
27.149 |
4.772 |
0.032 |
-0.241 |
1.182 |
1.073 |
1.302 |
|
NLM2, t=0 h |
fba1 |
22.377 |
|
|
|
|||
|
Control, YPD, t=0 h |
fap1 |
27.738 |
4.406 |
0.098 |
-0.607 |
1.523 |
1.137 |
2.039 |
|
Control, YPD, t=0 h |
fba1 |
23.331 |
|
|
|
|||
|
NLM2, t=3 h |
fap1 |
27.385 |
1.712 |
0.090 |
-3.301 |
9.855 |
7.543 |
12.875 |
|
NLM2, t=3 h |
fba1 |
25.673 |
|
|
|
|||
|
Control, YPD, t=3 h |
fap1 |
27.130 |
3.328 |
0.041 |
-1.685 |
3.215 |
2.846 |
3.631 |
|
Control, YPD, t=3 h |
fba1 |
23.802 |
|
|
|
|||
|
NLM2, t=6 h |
fap1 |
28.109 |
1.329 |
0.015 |
-3.683 |
12.847 |
12.296 |
13.423 |
|
NLM2, t=6 h |
fba1 |
26.779 |
|
|
|
|||
|
Control, YPD, t=6h |
fap1 |
24.808 |
1.093 |
0.021 |
-3.920 |
15.134 |
14.196 |
16.134 |
|
Control, YPD, t=6h |
fba1 |
23.715 |
|
|
|
|||
|
NLM2, t=9 h |
fap1 |
24.321 |
1.311 |
0.028 |
-3.702 |
13.015 |
12.388 |
13.673 |
|
NLM2, t=9 h |
fba1 |
23.010 |
|
|
|
|||
|
Control, YPD, t=9 h |
fap1 |
24.472 |
1.341 |
0.061 |
-3.672 |
12.747 |
11.442 |
14.200 |
|
Control, YPD, t=9 h |
fba1 |
23.131 |
|
|
|
Table 8 Results for the first analysis for Comparative C_T (〖∆∆C〗_T) after cell harvesting at 0 h, 3 h, 6 h and 9 h using NLM2 (0.125 g/mL (NH4)2SO4). The experiments were run in triplicate
|
Sample name |
Target |
Cт mean |
ΔCт mean |
ΔCт SE |
ΔΔCт |
2^(-) RQ |
RQ min |
RQ max |
|
reference sample |
fap1 |
26.834 |
5.112 |
0.036 |
0.000 |
1.000 |
0.726 |
1.378 |
|
reference sample |
fba1 |
21.722 |
|
|
|
|||
|
NLM3, t=0 h |
fap1 |
28.126 |
5.562 |
0.212 |
0.450 |
0.732 |
0.389 |
1.377 |
|
NLM3, t=0 h |
fba1 |
22.563 |
|
|
|
|||
|
Control, YPD, t=0 h |
fap1 |
27.683 |
4.024 |
0.033 |
-1.088 |
2.126 |
1.926 |
2.347 |
|
Control, YPD, t=0 h |
fba1 |
23.660 |
|
|
|
|||
|
NLM3, t=3 h |
fap1 |
27.810 |
2.997 |
0.085 |
-2.115 |
4.331 |
3.357 |
5.589 |
|
NLM3, t=3 h |
fba1 |
24.812 |
|
|
|
|||
|
Control, YPD, t=3 h |
fap1 |
27.392 |
3.472 |
0.071 |
-1.640 |
3.117 |
2.520 |
3.855 |
|
Control, YPD, t=3 h |
fba1 |
23.920 |
|
|
|
|||
|
NLM3, t=6 h |
fap1 |
27.868 |
2.073 |
0.046 |
-3.039 |
8.220 |
7.158 |
9.440 |
|
NLM3, t=6 h |
fba1 |
25.795 |
|
|
|
|||
|
Control, YPD, t=6h |
fap1 |
24.609 |
1.446 |
0.040 |
-3.667 |
12.699 |
11.256 |
14.327 |
|
Control, YPD, t=6h |
fba1 |
23.163 |
|
|
|
|||
|
NLM3, t=9 h |
fap1 |
27.498 |
1.513 |
0.012 |
-3.599 |
12.119 |
11.710 |
12.543 |
|
NLM3, t=9 h |
fba1 |
25.985 |
|
|
|
|||
|
Control, YPD, t=9 h |
fap1 |
28.863 |
1.652 |
0.085 |
-3.460 |
11.004 |
8.542 |
14.174 |
|
Control, YPD, t=9 h |
fba1 |
27.210 |
|
|
|
Table 9 Results for the first analysis for Comparative C_T (〖∆∆C〗_T) after cell harvesting at 0 h, 3 h, 6 h and 9 h using NLM3 (0.006 g/mL (NH4)2SO4). The experiments were run in triplicate
After the corresponding initial analysis, two additional replicates of qPCR were conducted for each nitrogen-limited medium and its respective controls. A total of 270 Ct values were obtained from 10 samples used in each run, including the reference sample, negative control, control samples grown in YPD, and experimental samples grown in NLM at different times. Figure 11 shows the average of the relative quantification results for the assays with NLM1, NLM2 and NLM3. The cDNA fold change was compared using the two-factor ANOVA, where the first factor is the time point and the second factor is the ammonium sulfate concentration. The null hypothesis was established as H0: μNLM1 = μNLM2 = μNLM3 for all time points. The F-value for the rows (ammonium sulfate concentration) was 6.57 with a p-value of 0.0308, which is less than 0.05. This result suggests that statistically there is a significant difference in Ct values among the samples exposed to different ammonium sulfate concentrations. Meanwhile, the F-value for the columns (Time Points) was 9.79 with a p-value of 0.0100, which is also less than 0.05. These results suggest that the observed differences are statistically significant and are unlikely to be due to chance. In conclusion, the null hypothesis can be rejected in this case because the obtained p-value is less than the established significance level (α = 0.05), suggesting that statistically there is also a significant difference in RQ values across the different time points, where independently both factors influence the cDNA fold change. Besides the abovementioned results from ANOVA, the results were also evaluated using the least significant difference (LSD). LSD is a post-test used to identify if the difference between the means of different groups is statistically significant.21 The results for the LSD analysis suggest that yeast exposure within the time points t=0 h/ t=6 h and t=0 h/ t=9 h causes a significant effect on the RQ. Meanwhile, the concentration of ammonium sulfate has no significant effect on the calculated RQ. These results suggest that the factor more critical for influencing the expression of the fap1 gene is when the yeast cell is exposed to an extended time to nitrogen-limited conditions than for limitation itself to nitrogen sources. The experimental results reflect that the fap1 gene responds better in the presence (but not in the absence) of ammonium ions because its induction or expression was best observed in ammonium-contained medium, in the following order: NLM1(0.25 g/mL) > NLM2(0.125 g/mL) > NLM3(0.006 g/mL). Another important aspect that should be mentioned is that the large error bars observed in the results may be associated with the use of cell disruption techniques (enzymatic and mechanical) when preparing the crude yeast extracts, or also with the dilutions performed. These differences in preparation techniques can introduce variability in the data, which is reflected in the error bars. According to the two-tailed t-test analysis, significant differences in the relative quantity (RQ) were found between YPD and NLM at 9 h (for experiments involving NLM1), and at 3 h (for experiments involving NLM2). In the case of NLM3, controls exhibited higher expression of the fap1 gene at 0 h (C3 > N3). This result suggests that the fap1 gene response can serve as a 'biomarker' in S. cerevisiae under stress conditions, observed in NLM1 (N1 > C1) at 9 h and NLM2 (N2 > C2) at 3 h.

Figure 11 Fold Change Profiles Over Time: Experimental and Control Samples. (N=3) cDNA obtained from cells grown in NLM1 (A), NLM2 (B) and NLM3 (C), and YPD medium for 0 h*, 3 h**, 6 h*** and 9 h****. Mean ± SEM is plotted. (A) *p < 0.933; **p < 0.059; ***p < 0.072; ****p < 0.043; (B) *p < 0.639; **p < 0.010; ***p < 0.926; ****p < 0.798; (C) *p < 1.32E-5; **p < 0.168; ***p < 0.346; ****p < 0.147. p values were determined by a two-tailed t-test.
It is also known that during the exponential phase, yeast cells tend to grow more rapidly, resulting in a higher accumulation of nucleic acids. In the case of NLM2 samples, a decrease in cDNA concentration was observed at the time point of 9 h. This decrease in cDNA concentration could be associated with yeast transitioning out of its exponential growth phase into the stationary phase, indicating the onset of cell death. The results also suggest that changes in yeast metabolism did not differ significantly in response to low concentrations of ammonium sulfate. All yeast-derived samples may have similar results because other proteins are being expressed, as demonstrated by the study of the yeast proteome in response to various nutrient limitations.22 The Kolkman’s research group explained that in response to a low ammonia supply, yeast cells induced the expression of proteins involved in the transport (Gap1p) and degradation (Dal7p, Put2p, Asp31p) of alternative nitrogen sources. Another cellular mechanism for nitrogen utilization involves amino acid recovery through protein turnover, which is mediated by the positive regulation of four independent, ubiquitin-dependent proteases. While FAP1 is not the focus of their study, Li’s research group suggests its potential role in the quality control process of proteins, particularly regarding ubiquitination.23 The statistical analyses generated from the experimental results support the idea that the expression of the fap1 gene increases over time in response to the change of the growth medium (i.e., NLM). When yeast cells are exposed to nitrogen-limited conditions, they tend to adapt quickly to all nitrogen sources available. Therefore, it is not a relevant factor to think that the concentration of ammonium sulfate is what yeast utilizes as a unique nutrient source to readjust its metabolism and survive because its proteome and transcriptome can modify to economize and reuse amino acids, leading to similar metabolic changes even with different concentrations of the nutrient (i.e., ammonium sulfate). Additionally, altering the growth environment modulates the expression ratio of different genes within each process, particularly those related to metabolism.24 This behavior seems to occur for the fap1 gene, in yeast, whose expression levels increased in all nitrogen-limited media (NLM) applied to our studies. Li’s research group has explained that the expression patterns observed for those yeast proteins involved in nitrogen utilization and protein turnover have shown to play a crucial role in adapting yeast to nitrogen-limited conditions and regulating cellular quality control processes.23 Their results also validate the possible involvement of the fap1 gene with protein turnover, as its mRNA (cDNA) levels increased when yeast cells were exposed to the nitrogen-limited media.
This research work focused on determining the expression levels of the fap1 gene in nitrogen-limited conditions by qPCR. The impact of nitrogen-limited media on the cDNA concentration (based on the expression levels of the fap1 gene) was less pronounced compared to the effect of time. Despite fluctuations in ammonium sulfate levels, the cDNA concentration consistently increased over time. These findings are relevant for understanding how yeast responds to diverse environmental conditions and adapts to variable nutrient availability. Furthermore, they offer valuable insights into the role of FAP1 underlying nitrogen-limited media and time responses, which could prove beneficial in biotechnological applications and enhance our understanding of fundamental biological processes in yeast.
This research was supported by the Puerto Rico IDeA Network of Biomedical Research Excellence, an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant no. P20GM103475.
The authors declared that there are no conflicts of interest.

©2025 Fajardo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.