International Journal of
eISSN: 2573-2889


Research Article Volume 8 Issue 1
1PG Department of Biotechnology KSG College of Arts and Science, Coimbatore, India
2PG and Research Department of Biotechnology Nandha Arts and Science, India
Correspondence: P Sagadevan, PG Department of Biotechnology KSG College of Arts and Science, Coimbatore, 641015, Tamilnadu, India
Received: April 19, 2025 | Published: May 7, 2025
Citation: Sagadevan P, Raghunath M, Nagarajan M, et al. Analyze the effects of A paniculata leaf extract in methanol on Candida albicans RFG1 gene expression. Int J Mol Biol Open Access. 2025;8(1):27-32. DOI: 10.15406/ijmboa.2025.08.00194
The gene expression studies showed decreased level of Rfg1 gene expression in C. albicans upon treatment with methanolic leaf extracts of A. paniculata. However, similar molecular studies on pure compounds from this plant may give strong evidences about the pharmacological potential of the plant and thus can be formulated as an effective drug for the treatment of infections caused by C. albicans. The bioassays on antifungal activity of the methanolic leaf extract of A. paniculata showed inhibitory effects against human pathogen C. albicans.
Keywords: Rfg1, A. paniculata, C. albicans
An essential part of India's healthcare system, the use of plants as medicine dates back thousands of years. Since the majority of medical professionals in India create and administer their own medications, this calls for appropriate documentation and study. More than 45,000 species of medicinal plants may be found in India, with the biggest densities found in the Eastern Himalayas, Western Ghats, and Andaman & Nicobar Island. Although there are about 3,000 officially recognized plants with therapeutic potential, traditional healers employ around 6,000.1 Today, a significant portion of the global populace relies on medicinal plants for their health concerns. While Western medicine has distanced itself from herbal practices, between 75% and 90% of rural communities around the globe still depend solely on herbal remedies for healthcare. In numerous countries, markets feature medicinal herbs alongside fresh produce.2 China stands out as the foremost nation in integrating traditional herbal medicine with a contemporary healthcare framework, resulting in a fusion of herbal treatments, acupuncture, and Western medical practices. China hosts extensive plantations dedicated to the cultivation of medicinal plants, providing a vast array of species for herbal practitioners; prescriptions are composed of precisely measured specific herbs instead of pills or ointments. In India, traditional healing systems have largely remained distinct from Western medicine. Beyond ayurvedic medicine, which has its roots in Hinduism, Unani medicine–originating from Muslim and Greek traditionsis another prevalent herbal practice in India. The resurgence of interest in medicinal plants has sparked a focus on herbal remedies among indigenous populations globally, particularly those residing in tropical rainforests. It is hoped that these explorations will enrich the world’s pharmacopoeia with new medicinal potentials before they disappear entirely.3
Andrographis paniculata is a seasonal herbaceous flora widely cultivated and traditionally utilized across southern Asia, China, and certain regions of Europe. In popular terminology, it is referred to as the king of bitter, having been effectively employed in traditional Asian herbal remedies for centuries. The notably bitter and distinct flavor of A. paniculata belongs to the Acanthaceae family. In heritage medicine, A. paniculata is extensively utilized to eliminate excessive body heat, expel toxins, ward off common colds, manage upper respiratory tract infections including sinusitis and fever,4 and serves as an antidote for snake and insect venoms.5 Flucanazole possesses fungistatic qualities and is commonly used to treat a variety of Candida albicans infections. However, there have been reports of increased resistance among clinical strains of Candida albicans.6 Thus, there is a pressing necessity to extract novel fungal agents, primarily from botanical sources, aimed at discovering new chemical compounds devoid of the aforementioned drawbacks.7 Numerous plant derivatives and essential oils display biological activity both in vivo and in vitro, validating the inquiry into traditional medicine centered on characterizing their antimicrobial properties.8 A. paniculata's bioactive compounds against Candida albicans are the focus of this study, along with their impact on controlling RFG1 gene expression, which is essential for the yeast-to-hypha transition in Candida albicans.
Collection of plant materials and identification
The leaves of Andrographis paniculata (Figure 1) were collected from several parts of Tamilnadu, India's Coimbatore district. The Botanical Survey of India in Coimbatore, Tamilnadu, confirmed the gathered foliage and assigned it the authentication number BSI/SRC/5/123/2013-14/Tech 1934. After being cleaned two or three times with tap water, the obtained A. paniculata plant foliage was rinsed with distilled water to remove any last bits of debris that could have stuck to the leaves. The foliage was then allowed to dry in the shade. A. paniculata leaf powder was then produced by processing the dried plant leaves using the cold percolation technique.
Plant description A. paniculata is a herbaceous flora belonging to the Acanthaceae family, indigenous to India and Sri Lanka. It is extensively grown across Southern and Southeastern Asia, where it serves medicinal purposes for infections and various ailments, often utilized prior to the advent of antibiotics. Primarily, the leaves and roots were harnessed for therapeutic effects. In Ayurvedic tradition, it is referred to as Kalmegh or Kalamegha, translating to "dark cloud". It is also called Bhui-neem, meaning "ground neem", as this petite annual herb exhibits a similarly intense bitter flavor reminiscent of the large Neem tree (Azadirachta indica). Commonly known as nilevembu or nilaveppu in Tamil Nadu and Kerala, it thrives upright, reaching heights of 30-110 cm in humid, shaded areas. The slender stem is dark green, square in cross-section, featuring longitudinal grooves and wings along the edges. The lanceolate leaves exhibit hairless blades that can measure up to 8 centimeters in length and 2.5 in width. Small flowers appear in spreading racemes. The fruit consists of a capsule approximately 2 centimeters long and a few millimeters wide, housing numerous yellow-brown seeds.
Scientific classification
Kingdom: Planta
Order : Lamiales
Family: Acanthaceae
Genus : Andrographis
Species: paniculata
Anti-candidal assay
Fungal culture
Clinical strains of Candida albicans were obtained from the Bioline Laboratory in Coimbatore, the PSG Hospital in Coimbatore, and the Amrita School of Medicine in Kochi.
Preparation of inoculum: To stimulate the culture, a loopful of it was added to 30mL of sabouraud dextrose broth in an Erlenmeyer flask, and it was then incubated for 24 hours at 37°C on a rotary shaker.
Germ tube test
One diagnostic technique used to differentiate Candida albicans from other yeast species is the germ tube examination. A fresh, sterile microfuge tube was filled with 0.5 mL (12 drops) of human blood serum. After that, a loopful of Candidal culture was added to the serum, and it was incubated for two to three hours at 35 to 37°C. The growth of the germ tube may be impeded by an overabundance of inoculum. A drop of the mixture was put on a spotless, grease-free slide and covered with a slip after incubation. The development of germ tubes–long, tube-like projections that protrude from the yeast cells was seen under a microscope in the wet mount.
Bioassay
The agar well diffusion technique was used to assess the crude extracts' antifungal efficacy. Using a sterile cotton swab, a suspension of Candida albicans was added to Sabouraud dextrose agar plates. A sterile cork borer was used to make 6 mm wells on the inoculation plates, which were then filled with the extracts. For twenty-four hours, the infected plates were incubated upside-down at 37 °C. The solvent used to reconstitute the extract was also utilized to create control samples. The plates were examined for zones of inhibition 24 hours later. The results were contrasted with fluconazole (150 mg/mL), a common antibiotic.
Determination of minimum inhibitory concentration (MIC) in human blood serum
Plant extracts were added after a loopful of culture was aseptically added to 500 μL of human blood serum in a sterile microfuge tube. A control was a serum infected with Candida albicans without any plant extract added. After that, the inoculation tubes were incubated for 24 hours at 37 °C. After spreading around 10 μL of the incubated sample onto a hemocytometer, the number of hyphal and yeast cells was counted using an optical microscope (45X).
Extraction from candida
Clinical samples of five different species of Candida were obtained from Amrita School of Medicine in Kochi, PSG Hospital in Coimbatore, and Bioline Laboratory in Coimbatore. These species include Candida albicans, Candida krusei, Candida glabrata, Candida parapsilosis, and Candida tropicalis. DNA was extracted by performing CTAB method of fungal DNA extraction.
Preparation of reagents
a). Alcohol isoamyl chloroform (24:1) 24 mL of chloroform and 1 mL of isoamyl alcohol, respectively.
CTAB extraction buffer
For 100 mL of 1 M stock with pH 7.5–10 mL and 0.1M Tris
1 g, 0.7M NaCl, 14 mL, and 1% CTAB (of 5M stock)
2 mL (10mM EDTA) (of 0.5M stock)
1 mL of 1% beta-mercaptanol
73 mL of waterc).
TE Buffer
Tirs.Hcl - 10mM
EDTA - 1mM
pH - 8.0
d). Ice Cold Isopropanol (or) Ethanol
Only after NaCl is added and the solution is heated to 65°C for a little while being vigorously stirred will the CTAB completely dissolve. Five milliliters of 18-hour-old Candidal cultures were pelleted for five minutes at 4°C at high speed. After adding 500 μL of CTAB extraction buffer, 15 μL of proteinase K, and 0.1 g of acid-sterilized glass beads to the tube, the mixture was forcefully vortexed for five minutes to break down the fungal cell wall. It was then incubated for thirty minutes at 65°C in a water bath. After cooling the tubes, an equivalent amount of chloroform isoamyl alcohol (24:1) was added, mixed by rocking, and centrifuged at high speed for 10 minutes at 4°C. After transferring the aqueous supernatant to a new, sterile microfuge tube, an equal amount of ice-cold isopropanol was added, mixed by inversion, and spun at high speed for ten minutes. After being cleaned with 70% ethanol, the pellets were allowed to air dry before being reconstituted in TE buffer for further examination.
Electrophoresis of genomic DNA
Reagent preparation
Tris Acetate Buffer (1X) (a) pH: 8.
Base for Tris: 242 mg
57.1 mL of glacial acetic acid
From 0.5 M EDTA to 100 mL. The amount was increased to 1000 milliliters. 10 mL of 50 X stock was diluted with 490 mL of distilled water to create 1X buffer.
Gel loading dye
0.025 grams of bromophenol blue 1X TAE buffer was used to get the volume up to 10 mL.
Ethidium bromide
10 mg of ethidium bromide and 0.2 mL of ethanol 0.8 milliliters of distilled water 1% Agarose 0.25g of Agarose was dissolved in 25mL 1X TAE buffer
Procedure
The agarose was completely dissolved by heating a 1% agarose gel. A well forming was then used to include, fully mix, and pour ethidium bromide (at a final concentration of 0.5 mg/mL) into the gel casting equipment. At room temperature, the gel was allowed to polymerize. The extracted genomic DNA was separated using horizontal electrophoresis in a tris acetate EDTA buffer for 45 minutes at 50 V. After thoroughly mixing the sample with bromophenol blue (in a 1:1 ratio), the specimens were cautiously added to the wells using the tip of a micropipette. Using a gel documentation system (Biorad, Italy), the bands were visible.
PCR amplification of Rfg1 gene
Using genus-specific primers (RFG1FP: 5' – TATCATCCTCCACCACCACA – 3' and RFG1RP: 5' – CTCCACCTCCACCTCCATTA – 3'), PCR was performed to screen the rfg1 gene. The anticipated amplicon size was 853 bp. A 10µL PCR reaction was carried out. 5µL of PCR master mix (2X) (Amplicon, India), 2µL of nuclease-free water, 1µL of each forward and reverse primer (10 pM), and 1µL of template DNA (50 ng) were used for each reaction. The response was carried out under the following circumstances.
Initial Denaturation: 5 minutes at 95 OC
Denaturation: 94 OC for 30 s
30 seconds at 51.1 OC for annealing
Extension: 72 OC for one minute
Final extension: five minutes at 75OC
Number of cycles: 30
Final custody: 4OC
Electrophoresis of PCR product
The product (10µL) and 5µL of loading buffer were separated by horizontal electrophoresis using a 1.5% Agarose (low EEO grade, HiMedia, India) gel enriched with ethidium bromide (final concentration 0.5 mg/mL) for two hours at 50V in tris-acetate EDTA buffer after amplification. An ultraviolet transilluminator was used to view the bands. The amplified products' sizes were determined by comparing them to a 500 bp DNA ladder. The gel documentation system was used to visualize the bands, and the outcomes were noted.
Treatment of C. albicans with A. paniculata
Candida albicans were inoculated into a series of centrifuge tubes using five milliliters of sabouraud dextrose broth. After that, methanolic leaf extracts of A. paniculata were put into each tube. The tubes were then incubated at 37°C for various durations of time (1, 6, 12, 24, and 48 hours).
Extraction of total RNA from tissues
After blending the cultured samples with one milliliter of trizol reagent, they were let to remain at room temperature for ten minutes. 200μL of chloroform and 0.1 g of acid-washed glass beads were then added, rapidly shaken, and allowed to sit at room temperature for two to three minutes. After centrifuging the mixture for 10 minutes at 4°C at maximum speed, the aqueous layer was carefully transferred to a separate tube and 500µL of isopropanol was added. After being inverted and allowed to sit at room temperature for five minutes, the tubes were centrifuged for twenty minutes at 12,000 rpm. After adding 1 mL of 80% ethanol, the mixture was centrifuged for 10 minutes at 4°C at 12,000 rpm, with the supernatant discarded. 100% ethanol was added to the pellet and spun for a further 10 minutes at 4°C at 12,000 rpm. The supernatant was then disposed of. The pellet was allowed to air dry before being resuspended in 30-60µl of water treated with DEPC (Diethyl Pyrocarbonate) and stored at -80°C for further examination.
RNA quantification and purity
Using a Cyberlab 100 UV/Vis spectrophotometer, the extracted RNA was measured spectrophotometrically by calculating the absorption ratio at 260 and 280 nm. The following formula was used to calculate the RNA concentration.
Concentration of RNA sample (μg/mL) = 40 × A260 × dilution factor (200)
Where, A260: Absorbance (in optical densties) at 260 nm,
40: Average extinction coefficient of RNA.
Synthesis of cDNA
In a microfuge tube, the following mixture was made. 4 µL of 5X cDNA synthesis buffer, 2 µL of dNTP mix, 1 µL of RNA primer, 1 µL of RT enhancer, 1 µL of reverse enzyme mix, 1–5 µL of template RNA, and 9 µL of nuclease-free water were used for each reaction. After carefully mixing the reaction mixture, it was immediately incubated for 30 minutes at 42 OC. After five minutes of incubation at 95 OC, the enzymes were cooled on ice to inactivate them.
RT-PCR
Transcriptase in reverse following treatment with extracts from A. paniculata, PCR was used to assess the Rfg1 gene's RNA expression levels in Candida albicans. For RT-PCR, a total of 10 µL reaction was performed. Five microliters of PCR master mix (2X) (Amplicon, India), two milliliters of nuclease-free water, one microliter of each of the forward and reverse primers (10 pM), and one microliter of template C-DNA were used in each reaction. For the reaction, the previously specified primers and experimental setup were used.
Electrophoresis of PCR product
Following amplification, a 1.5% Agarose gel (low EEO grade, HiMedia, India) loaded with ethidium bromide (final concentration 0.5 mg/mL) was subjected to horizontal electrophoresis for two hours at 50V in tris-acetate EDTA buffer (8). The sample (10µL) was further accompanied by 5µL of loading solution. An ultraviolet transilluminator was used to find the bands. By comparing the amplified products to a 500 bp DNA ladder, their dimensions were evaluated. The bands showed whether the Amplicon was present or not.
Bioassay
At several doses, including 62.5, 125, 250, and 500 mg/mL, the methanolic leaf extract of A. paniculata showed antifungal efficacy against Candida albicans (Table 1) (Figure 2). When 125 mg/mL was used, the inhibition zone's size was 19.0±0.4 cm. At the higher doses of 250 and 500 mg/mL, the zone size was 1.0 cm larger than it was at the 125 mg/mL level. Consequently, it may be said that the dose of 125 mg/mL shows the greatest degree of inhibition. The positive control was the antibiotic doxycycline.
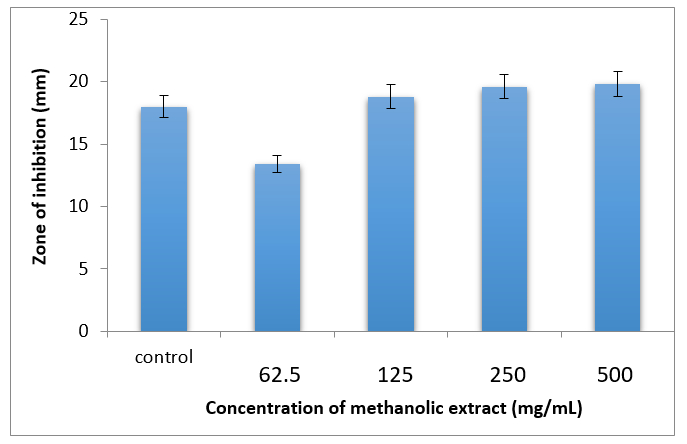
Figure 2 Graph showing zone of inhibition against Anticandidal activity against of methanolic leaf extract of A. paniculata.
|
Concentration of extract (mg/mL) |
Zone of inhibition (mm dia) |
|
Control (doxycyclin) |
14.0±0.0 |
|
62.5 |
13.0±0.5 |
|
125 |
19.0±0.4 |
|
250 |
20.0±0.5 |
|
500 |
20.0±0.4 |
Table 1 Anticandidal activity of methanolic leaf extract of A. paniculata
Germtube test
To determine the morphological differences of Candida albicans, the Germ Tube Experiment was carried out. While the cells grown in human blood serum formed elongate projections on the surface of blastospores, resulting in the development of germ tubes, the cells grown in sabouraud dextrose broth appeared as blastospores that multiplied by budding (Figure 3). According to this experiment, the organism changed from a yeast-like (non-virulent) to a hyphal-like (virulent) shape in response to changes in the environment and the availability of nutrients. Short, unsegmented germinating hyphae were the first signs of germ tubes. They were three to four times as long and half as wide as the parent cell from which they were derived. There was no restriction on the link between the cell and the germ tube. This narrow juncture distinguished buds and pseudo-hyphae from germ tubes. Different quantities of the plant extract were added to serum that had been infected with Candida albicans in separate tubes. By counting the quantity of blastospores and hyphal cells under an optical microscope (45X), the incubated samples were examined to determine the inhibitory impact on the organism developed in serum (Figure 4 & 5). The Rfg1 (Repressor of filamentous growth) gene, which has been found to be a virulence factor associated with the yeast-to-hypha transition in Candida albicans, was tested for presence in DNA extracted from five different species of Candida (C. albicans, C. krusei, C. tropicalis, C. glabrata, and C. parapsilosis). In Candida albicans, the PCR amplified product showed the Rfg1 gene at the expected length of 853 bp, however in other Candida species, it was not present (Figure 6).
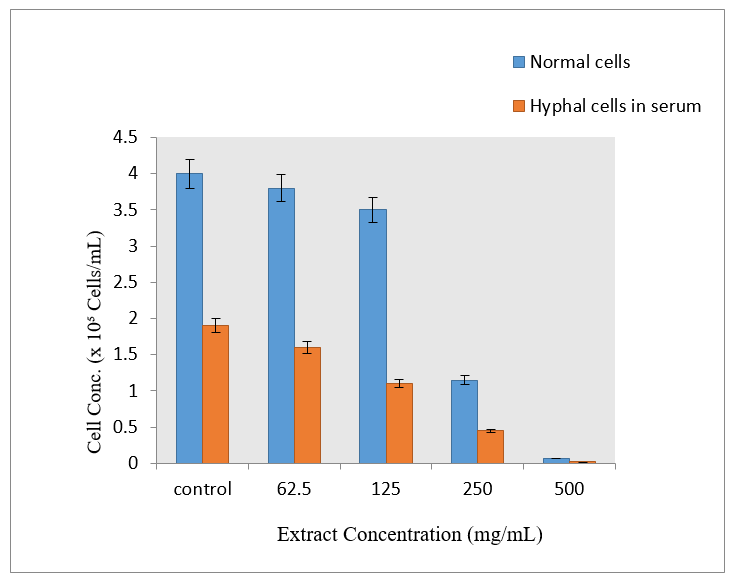
Figure 4 Determination of inhibitory activity of A. paniculata against C. albicans grown in human blood serum.
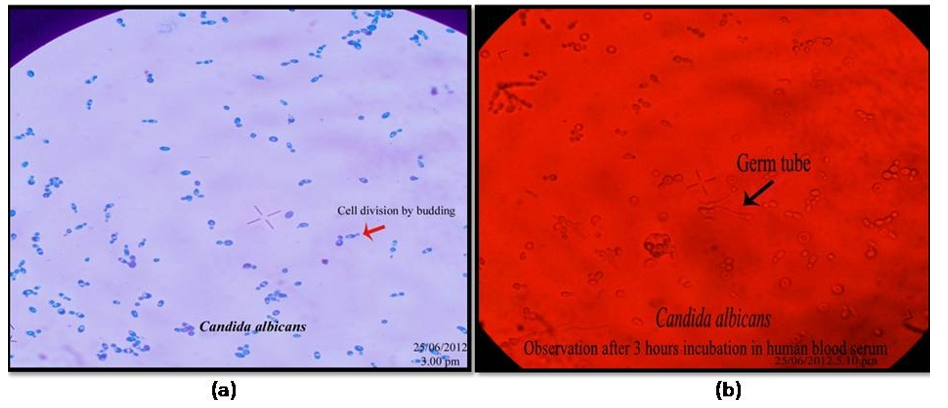
Figure 5 Microscopic observation showing blastospores (a) and germ tube/ hyphal cells (b) of C. albicans.
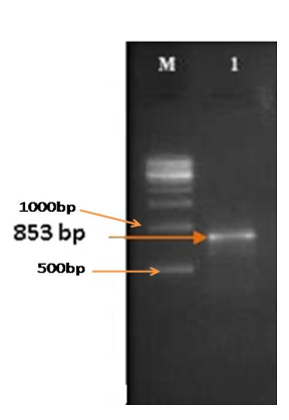
Figure 6 Screening of Rfg1 gene of Candida albicans, Lane M: 500 bp ladder; Lane 1: Amplicon Rfg1 gene of Candida albicans.
Expression of Rfg1 gene in Candida albicans after treatment with methanolic leaf extract of A. paniculata
RT (Reverse Transcriptase) PCR was used to evaluate the effect of methanolic leaf extracts from A. paniculata on the Rfg1 gene in C. albicans. Following treatment with A. paniculata, the C. albicans culture was incubated for different durations (1, 6, 12, 24, and 48 hours) and the expression levels of the Rfg1 gene were measured at each time point.The results showed that when treatment time increased from one hour to twenty-four hours, the expression of the Rfg1 gene decreased. After 48 hours of treatment, the gene was discovered to be re-expressed, indicating that the organism may have adapted to its surroundings and started to multiply because the extract concentration was either too low to stop growth or other factors may have promoted it (Figure 7).
Scientific interest in medicinal plants has recently increased due to the increased efficacy of plant-based drugs and rising apprehension about the side effects associated with traditional medicines. The ongoing emergence of drug-resistant microbes and the development of microbial pathogens in response to commonly used antimicrobials pose challenges to the effectiveness of currently available antimicrobial medicines. As a result, the search for new natural medicines continues to be a major path in the development of pharmaceuticals. The spread of multidrug-resistant bacteria is threatening the therapeutic efficacy of several medicines. Herbal remedies have long been used to treat a variety of infectious diseases. Because of their unmatched chemical diversity, natural substances–whether in their isolated forms or as standardized plant extracts offer countless opportunities for novel therapeutic candidates. Whether applied topically or taken orally, azole drugs and their derivatives continue to be the principal antifungal therapies for Candida-related illnesses. These drugs are known to have negative side effects, despite their great reputation for efficacy.9–12 Although fluconazole has fungistatic qualities and is commonly given for a variety of Candida albicans infections, there have been reports of increased resistance among clinical strains of the parasite.13 In order to find new chemical entities that get around these problems, it is imperative to find new antifungal drugs, especially from plant extracts.14
Gene expression activity of A. paniculata
Numerous studies have been carried out to examine the inhibitory effect of natural botanical chemicals on the levels of dangerous bacteria' gene expression.15 used real-time PCR to demonstrate the impact of licorice extract on the expression of the af1R gene and aflatoxin production in Aspergillus parasiticus, revealing that the extract decreased mycelial growth in A. parasiticus as the extract concentration rose. Numerous genes linked to virulence mechanisms are expressed during the complexly controlled hyphal growth of Candida albicans.16 RFG1, HGC1, VPS 11, and NOT4 are among the specific filament-induced genes that are essential for promoting hyphal growth. Results from17 demonstrated the importance of RFG1 in C. albicans' transformation from yeast to mycelial form. Building on these results, the current work evaluated Rfg1 gene expression levels after treatment with methanolic extracts of the plant across varying time intervals to show the antifungal qualities of A. paniculata against C. albicans. However, the activation of hyphal development in Candida albicans is also linked to other genes.18 The extraction and analysis of pure chemicals from this plant, as well as future studies into how the plant extract affects additional virulence genes, may provide important new information that supports the current findings. Nonetheless, the findings provide compelling molecular-level evidence in favor of earlier findings about A. paniculata's antifungal effectiveness. Furthermore, it is stressed that in order to fully understand the potential of these widely used medicinal herbs for the good of society, thorough research should be carried out.19
Gene expression studies showed that after C. albicans was treated with methanolic leaf extracts of A. paniculata, there was a decrease in Rfg1 gene expression. However, carrying out comparable molecular analyses on the plant's isolated chemicals would yield strong proof of the plant's pharmacological potential, opening the door for its development as a robust treatment for infections brought on by Candida albicans.
None.
The authors declared that there are no conflicts of interest.

©2025 Sagadevan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.