International Journal of
eISSN: 2576-4454


Research Article Volume 9 Issue 3
1Forest Research Centre (CEF), Instituto Superior de Agronomia, Universidade de Lisboa, Tapada da Ajuda, 1349-017 Lisboa, Portugal
10Centro de Investigação Interdisciplinar em Sanidade Animal (CIISA), FMV-UL, Av. da Universidade Técnica, 1300-477 Lisboa, Portugal
11AL4AnimalS, FMV-UL, Av. da Universidade Técnica, 1300-477 Lisboa, Portugal
2Unidade Estratégica de Investigação e Serviços dos Sistemas Agrários e Florestais e Sanidade Vegetal (UEIS-SAFSV), Av. da República, Quinta do Marquês, 2780-159 Oeiras, Portugal
3GREEN-IT Bioresources for Sustainability, ITQB NOVA, 2780-157 Oeiras, Portugal
4Unidade de Tecnologia e Inovação, INIAV—Instituto Nacional de Investigação Agrária e Veterinária, 2780-157 Oeiras, Portugal
5GeoBioTec - Geobiociências, Geoengenharias e Geotecnologias, FCT-UNL, 2829-516 Caparica, Portugal
6LEAF—Linking Landscape, Environment, Agriculture and Food Research Center, Instituto Superior de Agronomia, Universidade de Lisboa, 1349-017 Lisboa, Portugal
7Associate Laboratory TERRA, Instituto Superior de Agronomia, Universidade de Lisboa, 1349-017 Lisboa, Portugal
8Associate Laboratory TERRA, Instituto Superior de Agronomia, Universidade de Lisboa, 1349-017 Lisboa, Portugal
9Centro de Investigação Interdisciplinar Egas Moniz (CiiEM), Quinta da Granja, 2829-511 Monte da Caparica, Portugal
Correspondence: Catarina Esteves, Forest Research Centre (CEF), Instituto Superior de Agronomia, Universidade de Lisboa, Tapada da Ajuda, 1349-017 Lisboa, Portugal
Received: August 28, 2025 | Published: September 10, 2025
Citation: Esteves C, Menino R, Ramos AC. Supplementary addition of pig slurry and poultry manure as a source of soil organic matter: effect on tomato yield and quality. Int J Hydro. 2025;9(2):95-100. DOI: 10.15406/ijh.2025.09.00408
Nutrient-rich livestock byproducts have a rich organic matter (OM) composition, contributing to increased soil fertility and supporting circular agricultural systems. This study, with a duration of 5 months, assessed the complementation of conventional mineral fertilisation with either pig slurry (PS) or composted poultry manure (CPM) in processing tomato (Solanum lycopersicum L.) cultivation, to provide OM, a valuable resource for easily depleted Mediterranean soils. It was established a completely randomised field trial to evaluated effects on tomato yield, fruit quality [biometrics, colour, soluble solids content (SSC), titratable acidity (TA)], and bioactive compounds [total phenolic content (TPC), antioxidant activity (AOx), lycopene, and ascorbic acid]. Key findings showed that PS reduced yield by 17% (119 t ha⁻¹ vs 142–147 t ha⁻¹ in the control and CPM treatments), likely due to osmotic stress from elevated Na⁺, as evidenced by reduced plant dry matter (DM). However, all quality parameters remained consistent across treatments, with uniform fruit size (50.9 × 39.7 mm; 62.1 g), optimal industrial characteristics (a* = 20.3; SSC: 4.2 °Brix; TA: 0.6% citric acid), and stable bioactive profiles (TPC: 46.8 mg GAE/100 g; DPPH: 2664 μmol TE/100 g; lycopene: 5.4 mg/100 g). Notably, CPM performed comparably to the control in both yield and quality, while PS maintained quality despite reduced yield. These findings highlight the potential of livestock byproducts—particularly CPM—as effective complement to standard fertilisation, to provide OM to the soil, promoting sustainable tomato production and supporting circular economy goals without compromising quality.
Keywords: bioactive compounds, circular agriculture, composted poultry manure, fruit Yield, pig slurry, sustainable fertilisation, tomato quality
Modern agriculture faces the dual challenge of maintaining high productivity levels while minimising environmental impacts and conserving non-renewable resources.1 Although mineral fertilisers efficiently satisfy crop nutrient demands, their intensive use contributes to soil acidification, consequently impacting microbial community variation,2 depletion of soil organic nitrogen (N) and organic matter (OM),3 fossil reserves’ depletion, surface and groundwater contamination,4 and rising production costs, considering how volatile the market prices are for mineral fertilisers.5 Against this backdrop, the reutilisation of livestock byproducts presents a viable route to circular agriculture by closing nutrient cycles and diminishing reliance on external inputs and the costly disposal of agricultural wastes.
Livestock byproducts, such as animal manures, are rich sources of OM, N, phosphorus (P), and potassium (K), making them valuable fertilising materials and soil complements. Their use has been widely documented to enhance soil fertility by increasing soil organic matter (SOM), nutrient availability, pH, and microbial biomass and diversity.6 In addition to its chemical and biological benefits, manure application has a positive impact on soil physical properties, reducing bulk density and penetration resistance while improving porosity, water retention, and aggregate stability.7 By supplying OM, manure application supports carbon (C) sequestration and retention,8 as well as long-term soil fertility. These benefits are particularly relevant in Mediterranean agroecosystems, where low SOM levels and pronounced soil erosion are persistent challenges.9
Compared to mineral fertilisers, animal manures are often more cost-effective and locally available, particularly in regions with high livestock densities and manure surpluses,10 further reducing transportation costs. As of 2020, global manure production was estimated at 130 billion kg of N, with Europe contributing approximately 13 billion kg.11 These figures highlight the significant potential of manure to reduce dependence on mineral N fertilisers and imported nutrients within the European context, if used as replacements for said mineral fertilisers. On the other hand, if used as complements of OM, considering the rich OM content that ranges between 33 and 440 kg t-1 of manure or kg m-3 of slurry, depending on the type of animal and rearing,12 these organic materials serve as a useful complement to increase SOM and C sequestration. Nevertheless, the benefits of organic amendments such as manure, either in slurry form or composted, extend beyond vegetative growth: the origin and form of nutrient supply can markedly influence fruit quality attributes—size, colouration, firmness, and concentrations of bioactive compounds—that determine market value and health-promoting potential.13,14 Hence, comparative analyses of organic complementation versus mineral fertilisation only are indispensable for informing agronomic practices that are both ecologically sound and economically feasible, without compromising food safety or nutritional quality.
As a crop of global economic and nutritional significance, tomato (Solanum lycopersicum L.) occupies a unique position in modern agriculture. Its value stems not from caloric density – indeed, its low energy content aligns advantageously with current dietary recommendations – but rather from its complex matrix of bioactive compounds. The tomato’s distinction as the primary dietary source of lycopene, a carotenoid with demonstrated chemopreventive properties,15 has been well established across various cultivation systems. This phytonutrient profile is further enhanced by a characteristic phenolic composition, featuring chlorogenic acid derivatives and rutin, which contribute to its observed anti-inflammatory and metabolic regulatory effects. These nutritional attributes, however, are profoundly influenced by agricultural practices. Recent evidence suggests that cultivation parameters – particularly fertilisation strategies – may significantly modulate the expression of these valuable bioactive compounds while simultaneously affecting yield parameters.16
However, in areas of intensive livestock production, the safe disposal of eluents and other organic waste occurs with some frequency, without reliable information on their real effect. From this perspective, and based on the hypothesis that excessive organic fertilization may eventually be counterproductive for production in some specific contexts, in the present study were evaluated the effects of pig slurry (PS) and composted poultry manure (CPM), applied as OM complements, on tomato productivity or fruit quality, while maintaining basal mineral fertilisation in all treatments. A completely randomised field experiment with three replicates per treatment (mineral control, PS, CPM) quantified yield, leaf mineral composition, fruit biometrics (dimensions, mass), colour (CIELab), ripening indices (°Brix, titratable acidity), and bioactive compounds (total phenolics, DPPH/FRAP antioxidant activity, lycopene, and ascorbic acid).
Experimental set-up // Plant material and crop conditions
A field experiment was conducted in the Santarém (Portugal) region, using a soil cultivated with tomato (Solanum lycopersicum Mill., cv. H1015) intended for the processing industry. The soil was classified as a clay loam (45.1 %, 27.3% and 27.7% of sand, silt and clay, respectively), non-calcareous (1.6% CaCO3) soil and presented a slightly alkaline pH (7.9), low SOM content (1.72%), very high P (257 mg P2O5 kg-1) and magnesium (314.3 mg Mg kg-1) content, and a high concentration of K (113.3 mg K2O kg-1). Additionally, the soil also had a low N content (0.1%).
For the evaluation of the effects of OM complementation (applied based on N content, providing an additional 30 kg N ha-1) using pig slurry (PS) and composted poultry manure (CPM) compared to sole mineral fertilisation on tomato crops, it was established a complete randomized experimental design in triplicate, with three treatments: (i) mineral fertilisation (control, CTLR), (ii) PS supplementation, and (iii) CPM supplementation. In total, nine plots were established, each measuring 12 meters in width and 100 meters in length (Figure 1).
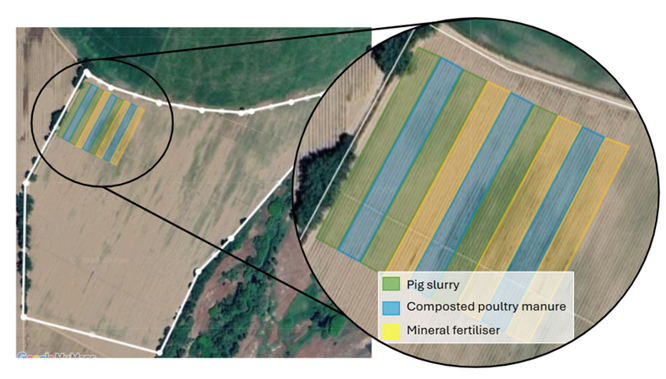
Figure 1 Aerial view of the experimental field, with details of the plots outlined for each treatment and the respective replicates.
Organic materials (PS and CPM) were analysed in duplicate, showing the following characteristics, for:
The mineral fertilisation was planned according to the farmers’ historical data, which is then based on regional recommendations. Prior to planting, 500 kg ha⁻¹ of Orgevit (4:3:2.5 NPK), 20 kg ha⁻¹ of Physiostart (28% P₂O₅), and 280 kg ha⁻¹ of Kallisop (50% K₂O) were applied to meet the crop’s initial N, P, and especially K requirements, given the tomato crop’s sensitivity to K deficiency. Throughout the growing cycle, fulvic acids were applied to enhance soil microbial activity, along with nematicides, calcium (Ca) supplements, root growth promoters, and additional K and other nutrients to fulfil the remaining crop nutrient requirements. Irrigation was carried out using the drip method, with drip tape semi-buried along the plant rows. Water management was performed by the farmer, based on crop water requirements and local climatological data.
Organic materials were applied on 19th of April. The slurry was distributed using a slurry tank equipped with a splash plate, while the composted manure was applied with a manure spreader. Application rates were calibrated beforehand by conducting a test run to determine the tractor speed required to empty a full tank evenly. This speed was then maintained during application to ensure that the calculated amount of organic material was applied to each plot area. Tomato planting was carried out on 20th of April 2024, and the fruits were harvested at the end of the cultural cycle, on the 7th of August 2024.
Sampling and analytical methods
During the experiment, two types of plant material were sampled: (i) leaves, collected during the inflorescence development stage, consisting of the youngest fully expanded leaf adjacent to the apical inflorescence, and (ii) fruits, harvested at the end of the growing season from each treatment plot (3 × 1 m within the row). Leaf samples were used to assess plant nutrient status following national recommendations for leaf analysis for tomato production,17 while fruit samples were evaluated for yield (t ha⁻¹), biometric traits (size and mass), CIELab colour parameters, ripeness indices [soluble solids content (SSC) and titratable acidity (TA)], and bioactive composition [total phenolic compounds (TPC) and antioxidant activity (AOx) by the DPPH and FRAP assays].
Foliar analysis
For foliar mineral analysis, 30 leaves per treatment, and replicate, were collected, rinsed with deionised water, and oven-dried at 65°C till constant weight (~48 h), and then ground to pass through a 0.5 mm sieve using a bench mill for subsequent chemical analysis. Dry weight (DW) was evaluated by weighing the dry material. Plant total nitrogen (TN) was determined following the macro Kjeldahl method, as described by Horneck and Miller.18 Total leaf P, K and sodium (Na) contents were determined after incineration in a muffle furnace at 450 °C, followed by acid digestion with 3 M HCl and analysed by inductively coupled plasma optical emission spectrometry (ICP-OES). All elements are reported to the DW at 105°C.
Tomato fruit yield
Fruit yield was assessed when the majority of tomatoes were visibly ripe, on the 7th of August 2024. For each plot, yield data were obtained from a composite sample consisting of three subsamples, each from 1 m2, randomly collected from within the plot to ensure representativeness.
Biometric and CIELab Colour parameters
For each treatment, four fruits were randomly selected from the composite sample collected in each treatment plot. Fruit length and height were measured using an electronic digital calliper (Powerfix Profi, Neckarsulm, Germany), and fruit weight was determined using an electronic balance (PS 3500/C/1, Radom, Poland). A colourimeter (Minolta CR-300, Osaka, Japan) was used to evaluate the samples’ colour by measuring L* (0: black; 100: white), a* (−60: green; +60: red), and b* (−60: blue; +60: yellow) parameters (C illuminant, second observer). The instrument was calibrated using a white tile standard (L* = 97.10; a* = 0.19; b* = 1.95). The hue angle (h°) was calculated as follows: h° = atan2(b, a) × (180/π) with subsequent adjustment to the range [0°, 360°) by adding 360° to negative values.
Ripeness parameters
Analyses were performed on the same four fruits per plot. Titratable Acidity (TA) was quantified via automated potentiometric titration (Hanna Instruments, Model HI-84532-02, Woonsocket, Rhode Island, USA) using 5 g of homogenised fruit tissue diluted in 45 mL of deionised water. Results were expressed as grams of citric acid equivalents per 100 g fresh weight (g CA/100 g FW; mean of triplicate determinations). The soluble solids content (SSC) was determined using a digital refractometer (Atago Palette PR-201, Tokyo, Japan), calibrated with distilled water. Measurements were performed on extracted fruit juice (triplicate), with results reported in °Brix units.
Bioactive composition
Analyses were performed on the same four fruits per plot. The hydrophilic fractions, used to determine the total phenolic content and antioxidant activity, were prepared from a 1:2.25 (m:v) mixture of the sample with methanol (100%; Honeywell, Charlotte, NC, USA) homogenised at 20,000 rpm for 1 min (Polytron UltraTurrax T 25 basic, IKA-Werke, Staufen, Germany), and incubated for 20 min in an ultrasonic bath (Sotel Branson 2200 Ultrasonic Cleaner, Bayern, Germany). Centrifugation was followed at 7000 rpm, for 20 min, at 4 °C (Sorvall RC5C, SS34 rotor, Sorvall Instruments, Wilmington, DE, USA), and the supernatant was collected and stored at −20 °C (Large Upright AEG OKO_ARCTIS Freezer, Los Angeles, CA, USA) until analysis.
The total phenolic content (TPC) was determined according to Swain et al.19 with modifications. Briefly, 2400 µL of distilled water, 150 µL of extract, and 150 µL of 0.25 M Folin-Ciocalteu reagent (Sigma-Aldrich, USA) were mixed and stirred, followed by the addition of 300 µL of 1 M sodium carbonate (Merck Millipore, USA). After incubation in darkness, at room temperature, for 2 hours, absorbance was measured at 725 nm using a Jasco V-530 UV/Vis spectrophotometer (Tokyo, Japan), with results expressed as milligrams of gallic acid equivalents per 100 g fresh weight (mg GAE/100 g fw).
Antioxidant activity was assessed via two complementary methods: i) DPPH assay, followed by Arnao et al.20 and Brand-Williams et al.21 with modifications, wherein 150 µL of extract was combined with 2850 µL of DPPH solution (TCI Chemicals, Belgium), and incubated under dark conditions at room temperature, for 2 hours, prior to absorbance measurement at 515 nm. Results were expressed as micromoles of Trolox equivalents per 100 g fresh weight (µmol TE/100 g fw); ii) FRAP assay adapted from the protocol of Thaipong et al.,22 utilising a FRAP reagent stock solution comprising 0.3 M sodium acetate buffer (pH 3.6; Sigma-Aldrich, USA), 10 mM TPTZ solution (Alfa Aesar, USA), and 20 mM ferric chloride solution (Sigma-Aldrich, USA) in a 10:1:1 ratio. A mixture of 0.2 mL extract and 1.8 mL FRAP reagent was incubated at room temperature for 5 minutes before spectrophotometric reading at 593 nm. Results were quantified as millimoles of ferrous sulphate equivalents per 100 g fresh weight (mmol FeSO₄·7H₂O/100 g fw).
Lycopene extraction was performed according to Berra.23 Tomato samples (2 g) were extracted with 50 mL of hexane:acetone: ethanol (50:25:25, v/v/v) on an orbital shaker (New Brunswick Scientific Co. Inc., New Jersey, USA) for 30 min. Subsequently, 15 mL of deionised water was added to the extract, and the mixture was phase-separated. The upper hexane layer (10 mL), containing lycopene, was transferred to a round-bottom flask and dried using a rotary evaporator (Heidolph, Laborota 4001, Germany) at 68 °C. The residue was resuspended in 8 mL of a tetrahydrofuran:acetonitrile: methanol (15:30:55, v/v/v) mixture for HPLC analysis. Lycopene was quantified using an HPLC system consisting of a Waters Alliance 2695 separation module (Waters, Milford, USA) and a Photodiode Array Detector (Waters 996, Waters, Milford, MA). Chromatographic separation was performed in reverse phase on a YMC Carotenoid column, 250x4.6 mm, S-5μm (YMC CO, LTD, Japan), at room temperature, using an elution gradient with a mixture of methanol: water (75:25, v/v) as solvent A and ethyl acetate as solvent B. Lycopene was detected at 470 nm and quantified based on an external calibration curve of peak area versus concentration. Results expressed as mg lycopene per 100 g fresh weight.
For ascorbic acid extraction, tomato samples (5 g) were weighed into a 50 mL volumetric flask and brought to volume with a 2% (w/v) aqueous metaphosphoric acid solution. The flask was shaken and then allowed to stand for 15 min, protected from light. The extract was filtered through a 0.45 μm nylon syringe filter (Acrodisc), and 20 μL of the filtrate was injected into the HPLC system. Ascorbic acid was quantified using an HPLC system consisting of a Waters Alliance 2695 separation module (Waters, Milford, USA) and a Photodiode Array Detector (Waters 996, Waters, Milford, MA). Separation was performed under isocratic conditions on a Spherisorb ODS2 C18 column (250 x 4.6 mm, 5 μm, Waters, Milford, USA) at room temperature. The mobile phase consisted of a dibasic potassium phosphate solution added with trimethylammonium bromide, with a flow rate of 1.0 mL/min. Ascorbic acid was detected at 296 nm and quantified using an external calibration curve of peak area versus concentration. Results expressed as mg ascorbic acid per 100 g fresh weight
Statistical analysis
Data were subjected to analysis of variance (two-factor factorial ANOVA) using StatisticaTM v. 8.0 Software from StatSoft.24 Statistically significant differences (p < 0.05) between samples were determined according to Tukey’s HSD test.
Production yield response to fertilisation regimes
Results indicated that the application of PS (approximately 119 t ha-1) significantly reduced tomato yield (Figure 2) by approximately 17% compared to the CTRL and CPM treatments (142 and 147 t ha-1, respectively). This decline was somewhat unexpected, considering that PS was applied as a complement. However, this negative impact on fruit production may be attributed to the high Na content of the slurry, as reflected by the significantly higher Na concentration observed in leaf tissue in this treatment compared to CTRL (Table 1). Tomato is considered moderately sensitive to salinity stress,25 and the elevated Na levels in the leaves suggest that salinity may have induced osmotic stress, impairing water uptake by the plant roots. The reduction in leaf DM in the PS treatment supports this interpretation, as lower DM under saline conditions is often associated with osmotic stress.26
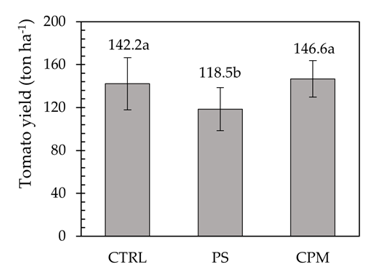
Figure 2 Tomato yield under different fertilisation regimes: mineral-fertilised control (CTRL), pig slurry application (PS), and composted poultry manure application (CPM). Identical letters (a, b) in the same column denote non-significant differences (Tukey’s HSD test). Values represent means ± standard deviation
Although no significant differences were observed between treatments for leaf TN, P, or K contents (Table 1), there was a consistent trend toward lower concentrations of these nutrients in the PS treatment. This pattern may reflect a slight reduction in nutrient uptake efficiency due to compromised water absorption under saline conditions, as water availability directly influences soil–plant nutrient transport.27 Therefore, complementation with PS did not appear beneficial for the farmer under the present experimental study’s conditions.
|
Treatments |
DM (%) |
TN (% DM) |
P (% DM) |
K (% DM) |
Na (% DM) |
|
CTRL |
94.13a ± 0.15 |
4.34a ± 0.18 |
0.28a ± 0.02 |
3.23a ± 0.31 |
0.61b ± 0.07 |
|
PS |
93.50b ± 0.10 |
4.05a ± 0.10 |
0.25a ± 0.01 |
2.71a ± 0.40 |
0.77a ± 0.06 |
|
CPM |
93.93a ± 0.15 |
4.25a ± 0.27 |
0.26a ± 0.03 |
2.91a ± 0.36 |
0.62ab ± 0.05 |
Table 1 Leaf dry matter (DM) and nutrient concentrations — total nitrogen (TN), phosphorus (P), potassium (K), and sodium (Na) — in tomato plants grown under different fertilisation regimes: mineral-fertilised control (CTRL), pig slurry application (PS), and composted poultry manure application (CPM). Values are expressed on a dry matter basis (mean ± standard deviation)
In contrast, the addition of CPM did not negatively affect tomato yield, which remained comparable to the mineral control. This suggests that CPM not only provides a valuable source of plant nutrients but may also improve soil structure and OM content without introducing excess salinity. Its lower Na content, albeit statistically similar to PS, likely contributed to the more favourable physiological conditions observed, as reflected in similar leaf DM and nutrient concentrations compared with CTRL. Thus, CPM appears to be a more suitable organic complement than PS for sustaining tomato yield while contributing to nutrient recycling. Different superscript letters within a column indicate significant differences between treatments according to Tukey’s HSD test (p < 0.05); identical letters denote no significant differences.
Fruit quality response to fertilisation regimes
Mean values for the biometric and physicochemical attributes of tomato fruit samples under different fertilisation regimes: mineral-fertilised control versus by-product complementation (PS and CPM) are presented on Table 2. Tomato fruits complemented with organic amendments—either PS or CPM—exhibited biometric and physicochemical quality parameters statistically equivalent to the mineral-fertilised control.
|
Quality attribute |
CTRL |
PS |
CPM |
|
Biometric parameters |
|||
|
Mass (g) |
54.67a ± 19.66 |
62.37a ± 9.82 |
63.30a ± 11.79 |
|
Length (mm) |
49.40a ± 8.93 |
50.92a ± 3.11 |
52.41a ± 3.95 |
|
Height (mm) |
38.43a ± 6.76 |
40.33a ± 4.50 |
40.36a ± 3.59 |
|
CIELab colour |
|||
|
L* |
33.26a ± 3.84 |
34.76a ± 4.15 |
34.76a ± 4.23 |
|
a* |
21.39a ± 4.38 |
19.42a ± 5.06 |
20.01a ± 4.67 |
|
b* |
26.49a ± 3.37 |
26.19a ± 4.63 |
26.57a ± 5.49 |
|
SSC (°Brix) |
3.93a ± 0.25 |
4.27a ± 0.12 |
4.40a ± 0.17 |
|
Titratable acidity (% citric acid) |
0.60a ± 0.04 |
0.61a ± 0.14 |
0.67a ± 0.09 |
|
Total phenolic content |
48.19a ± 1.13 |
40.60a ± 2.41 |
51.63a ± 6.64 |
|
(mg gallic acid eq./100 g) |
|||
|
Antioxidant activity |
|||
|
DPPH |
2529.60a ± 909.48 |
2181.82a ± 706.91 |
3280.97a ± 635.35 |
|
(µmol trolox eq./100 g) |
|||
|
FRAP |
3.64a ± 0.16 |
3.28a ± 0.47 |
4.94a ± 0.52 |
|
(mmol FeSO4•7H2O/100 g) |
|||
|
Lycopene content (mg/100g) |
5.09a ± 0.99 |
5.87a ± 0.71 |
5.39a ± 1.15 |
|
Ascorbic acid content (mg/100g) |
20.18a ± 1.66 |
18.83a ± 0.57 |
19.63a ± 0.87 |
Table 2 Biometric and physicochemical attributes of tomato fruits under different fertilisation regimes: mineral-fertilised control (CTRL), pig slurry application (PS), and composted poultry manure application (CPM). Values represent means ± standard deviation (expressed on a fresh weight basis)
Identical superscript letters (a, b) denote non-significant differences (Tukey’s HSD test). Key attributes, including average fruit mass (54.67–63.3 g), soluble solids content (4.2 ± 0.2 °Brix), titratable acidity (0.6 ± 0.1% citric acid), and chromatic intensity (a* ≈ 20.3), showed no significant differences across fertilisation regimes. Fruit morphology (length: 50–52 mm; height: 38–40 mm) was similarly consistent, meeting mechanical harvesting requirements. These findings demonstrate that partial organic fertilisation using CPM or PS maintains critical quality parameters within typical ranges for processing cultivars. Soluble solids values align with the standard industrial range (3.5–6.0 °Brix;,13,28), ensuring adequate sugar content for processing. Titratable acidity corresponds to conventional values (0.3–0.8%), while the sugar-to-acid ratio (≈7) reflects an optimal balance for processed products.13 The intense red hue (a* > 20) confirms optimal ripeness and adheres to CIELab standards for industrial cultivars.29 This consistency aligns with Gao et al.,16 who reported that strategies incorporating organic amendments preserve essential quality traits in processing tomatoes.
Lycopene concentrations (5.09–5.87 mg/100 g FW) fell within typical processing cultivar ranges (4–10 mg/100 g) and exceeded the premium threshold for tomato paste (>5 mg/100 g, according to Park et al.30 and Pék et al.29). Notably, the highest lycopene concentration was observed under PS treatment, although not statistically significant (p > 0.05). This finding contrasts with reports of lycopene loss in salt-sensitive genotypes under severe salinity31 but aligns with studies showing increased lycopene in response to moderate stress. The most plausible mechanistic explanation for this stability—or even enhancement—under PS-induced saline conditions is linked to stress-induced ethylene biosynthesis. As proposed by Wu and Kubota,32 osmotic and/or salt stress can trigger ethylene production, which in turn acts as a central signaling hormone upregulating the lycopene biosynthesis pathway. While the exact biological mechanisms are not fully elucidated, this ethylene-mediated response offers a compelling explanation for the maintained lycopene levels in our PS treatment, despite the signs of osmotic stress (e.g., reduced yield and leaf DM). Furthermore, the organic matrix of PS may have provided a mitigating effect, preventing the stress from reaching a severity threshold that would otherwise degrade fruit quality. This underscores the complex interplay between stress severity, plant physiology, and the form of nutrient supply in determining final fruit composition.
Ascorbic acid content (18.8–20.2 mg/100 g FW) aligned with open-field cultivation expectations (15–25 mg/100 g;14) but remained 20–40% lower than in protected environments, where reduced UV exposure and stable temperatures enhance accumulation,34 underscoring environmental factors’ dominance over fertilisation in determining vitamin C levels. Total phenolics (40.6–51.6 mg GAE/100 g FW) and antioxidant activity (FRAP: 328–494 mmol Fe²⁺/100 g FW; DPPH: 2182–3281 μmol TE/100 g FW) aligned with typical values reported for processing tomatoes under conventional or supplemented regimes.28,32,33,34
From an agro-environmental perspective, CPM and PS present distinct trade-offs. CPM offers circular economy benefits by enhancing soil organic C,9 but it exerts a limited influence on bioactive compounds. PS provided effective N delivery and lycopene stability under saline conditions; however, it reduced yield by 17% and increased the risk of Na accumulation. Blending PS with composted OM and implementing controlled irrigation could mitigate these limitations. Collectively, organic complementation preserves the physicochemical and functional quality of processing tomatoes to industrial standards, affirming its viability as an agroecological transition strategy without compromising key processing attributes.
Beyond yield and fruit quality parameters, the adoption of livestock byproducts as fertilisers offers critical agroecological advantages. Both PS and CPM contribute to the replenishment of SOM—a priority in Mediterranean soils that are inherently poor in organic C.9 This enhancement improves soil structure, water retention, and microbial diversity,7 while concurrently addressing waste management challenges in intensive livestock regions. By diverting these byproducts from costly or environmentally risky disposal routes (e.g., landfill, uncontrolled spreading), their agricultural reuse epitomises circular economy principles, closing nutrient loops at the farm scale.10 Although the PS application here incurred yield penalties under saline conditions, its integration with composted OM could mitigate risks while retaining SOM benefits.
From a long-term perspective, the repeated application of CPM is likely to enhance soil organic C stocks, improve microbial diversity, and sustain yield without salinity risks, thereby supporting resilient agroecosystems.9 In contrast, while pig slurry (PS) offers immediate nutrient availability and lycopene preservation, its continued use may exacerbate soil salinity and Na accumulation, necessitating integrated management strategies such as blending with low-Na amendments or using controlled irrigation to leach excess salts. Future studies should evaluate multi-year applications to assess cumulative effects on soil health, nutrient cycling, and tomato quality under Mediterranean conditions.
In conclusion, organic matter complementation to mineral fertilisation reliably maintains the commercial quality (size, colour, flavour) and sustains nutraceutical content of processing tomatoes within established industrial standards under the studied conditions, while promoting sustainable nutrient cycling. Further enhancements in bioactive compounds may require complementary strategies such as elite cultivar selection, fully organic regimes, or refined environmental stress management. Composted poultry manure presents a lower-risk circular solution, whereas pig slurry requires effective salinity mitigation to prevent yield compromise and enable its valorisation. This approach demonstrates significant circularity value by converting livestock waste into agronomic resources and elevating SOM in degradation-vulnerable Mediterranean agroecosystems. Future research should prioritise full organic substitution trials and develop cost-effective desalination approaches for the valorisation of pig slurry.
This research was supported by (1) the LivingLab - Effluents and coproducts of the livestock activity project funded by PRR (PRR-C05-i03-I-000218), (2) CEF, funded by FCT, project reference UIDB/00239/2020 of the Forest Research Centre, DOI 10.54499/UIDB/00239/2020; (3) LEAF (Linking Landscape, Environment, Agriculture and Food Research Unit), funded by FCT (UIDB/04129/2020);.
The author declares there is no conflict of interest.

©2025 Esteves, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.