International Journal of
eISSN: 2381-1803


Research Article Volume 14 Issue 6
1Department of Physiology and Biochemistry, University of Nyala, Sudan
2Institute of Molecular Biology, University of Nyala, Sudan
3Biomedical Research Institute, Darfur University College, Sudan
4Department of Clinical Medicine, Medical and cancer Research Institute, Sudan
5College of Veterinary and Animal Science, the Islamia University, Pakistan
6Department of Pharmacy, University “G. d’Annunzio” of ChietiPescara, Italy
7Department of Biology, Selcuk University, Konya, Turkey
Correspondence: Abdelkareem A. Ahmed, Department of Physiology and Biochemistry, Faculty of Veterinary Science, University of Nyala, Nyala, 583, Sudan
Received: February 24, 2021 | Published: December 15, 2021
Citation: Ahmed AA, Essa MEA, Ahmed H, et al. Gum Arabic modifies tissue heat shock proteins associated with decrease in oxidative stress and 11 beta-Hydroxysteroid dehydrogenase type I in mice. Int J Complement Alt Med. 2021;14(6):329-336. DOI: 10.15406/ijcam.2021.14.00580
Heat shock proteins (HSPs) are known to plays crucial roles in cellular cytoprotectant and are essential protein for the cells to boost toleration for pathogenic and environmental adaptation conditions. Gum Arabic (GA, Acacia senegal) works as a dietary fiber that improves antioxidant capacity. Yet, the effects of the GA on HSPs and its association with 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) have not been reported. In the present study, 20 female CD-1 mice of 90days old were randomly divided into two groups (n=10 of each group). Control group and GA group provided GA in the form of drink (10% w/v) for 15weeks. The treatment of GA significantly (P<0.05) reduced food intake and body weight body associated with decreased blood glucose and corticosterone levels. Similarly, the treatment of GA significantly (P<0.01) increased hepatic, muscle and cardiac HSP72 and HSP90 contents associated with increased activities of catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPx) compared control group. In addition, the treatment of GA significantly (P<0.05) decreased tissue MDA correlated with decreases in plasma total cholesterol, LDL-c concentrations whereas, increased HDL-c concentrations compared to the control group. However, the treatment of GA did not affect cardiac and muscle HSP 25 protein contents. The treatment of GA significantly (P<0.05) suppressed hepatic and cardiac 11β-HSD1 mRNA expression compared to control. On the other hand, the treatment of GA significantly (P<0.05) increased hepatic11β-HSD2 mRNA expression compared to control. In conclusion, GA may offer potential for improving the antioxidant defense against tissue damage.
Keywords: gum arabic, liver, mice, 11β-hydroxysteroid dehydrogenases, heat shock protein
Heat shock protein (HSP) is known to plays a vital role as cellular cytoprotectant1 and molecular chaperone.2 It is an essential protein for the cells to boost tolerance for environmental adaptation 3 or pathogenic conditions in various organ,4 depending on its capacity to assist fold newly synthesized protein and its degradation5 under normal and stressful condition.6 It has reported that the HSPs are reduced in disease states associated with insulin resistance7 and aging.8 And the induction of HSPs has been asoociated with decrease oxidative stress,9 inhibit inflammatory pathways,10 and improve metabolic status in skeletal muscle.11 Therefore, HSPs have the potential to function as an essential defense system against type 2 diabetes development.12
Data from previous studies showed that HSPs expression correlates with muscular oxidative capacity13 and their expression levels were significantly higher in the more oxidative soleus muscle when compared with the fast-twitch extensor digitorum longus or epitrochlearis muscles.14 Increasing evidence has shown that the humid heat stress causes substantial damage to human health and the cardiovascular system has been proposed to be the main target of heat stress,15-17 which consequences in severe cardiovascular diseases.18
The apoptosis of cardiomyocyte has been reported to play a critical role in the pathologenesis of cardiovascular diseases 19 including atherosclerosis,20 cardiac dysfunction,21 myocardial infarction,19 and heart failure.22
There cross link between HSPs and several types of diseases including insulin resistant, and type 2 diabetes millets (T2DM).23 In T2DM patients, the expression of HSPs was obviously changed24 and the T2DM patients muscle biopsies showed considerably lower mRNA expression of Hsp72 compared with those non-diabetic patients.25 In addition, a marked relationship between Hsp72 mRNA expression and insulin enhanced glucose uptake was observed in hyperinsulemic-euglycemic patient.26 Moreover, it was reported that skeletal Hsp72 mRNA expression decreased in T2DM patient which was consistent with skeletal Hsp72 protein expression.27 Furthermore, early assumption considered that the expression of Hsp72 may influence insulin sensitivity via a direct interaction with glucose transporter 4 (GLUT4).28 However, other reports revealed no changes in GLUT4 gene mRNA expression in diabetic patients compared with controls.29 Gum Arabic (GA) from Acacia seyal and Acacia senegal, is dried sticky exudate that contains soluble dietary fiber.30 However, the effects of GA on tissue HSPs have not been reported. In addition, it is less clear whether GA may modify liver, muscle and heart HSP contents in mice. Therefore, in the present study, we administered 10% of GA to mice to elucidate our hypothesis that the treatment of GA may change tissue HSPs contents and these changes may be associated with plasma CORT levels and 11β-Hydroxysteroid dehydrogenase type I and type II mRNA abundant in the mice tissues.
Experimental design and animal treatment
Forty female CD-1 mice of 90 days old female were obtained from Sudanese National Research Center, Khartoum, Sudan and housed at the Department of Toxicology, Faculty of Veterinary Medicine, and University of Khartoum in plastic cages (each containing 5 mice) in a room kept at 25 C with a 12-h light and dark cycle. The mice were allowed to access freely to a commercial pelleted diet for the adaptation and drinking water throughout the experiment at least for one-week days. After one week of adaptation period, the mice were divided into two groups. Control group (n =30) which was fed mouse standard diet, and treated group (n =30) fed mouse standard diet containing 10% of GA. GA group was provided drinking water containing GA while control group was given tape water. These mice received 0.5% of GA aqueous solution as drinking water for 7days, and then 10% solution for further 15 weeks consecutively. The control group was remained on the same drinking water as in the acclimatization. Body weight and food consumption were recorded during the period of the experiment. By the end of the experiment, the mice were killed. Liver, heart and muscle samples dissected and weighed. The tissue samples were collected and stored at-80 ◦C.
Blood lipid profile and glucose
Blood glucose, plasma total cholesterol, triglycerides (TG), low density lipoprotein (LDL), very low density lipoprotein (VLDL), and high density lipoprotein (HDL) were measured using commercially assay kits (Nanjing Jiancheng Bioengineering Company, Nanjing, China), according to the manufacturers’ instructions.
Plasma CORT measurement
Plasma corticosterone concentration was determined using an enzyme immunoassay. Corticosterone in 5μl plasma and 195μl water was extracted with 4 ml dichloromethane, re-dissolved in phosphate buffer and given in triplicate in the enzyme immunoassay. The dilution of the corticosterone antibody (Chemicon, Temecula, CA, USA; cross-reactivity: 11-dehydrocorticosterone 0.35%, progesterone 0.004%, 18-OH-DOC 0.01%, cortisol 0.12%, 18-OHB 0.02% and aldosterone 0.06%) was 1:8000. Horseradish peroxidase (1:400,000) linked to corticosterone served as the enzyme label and ABTS [2,2_-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid)] as the substrate. The concentration of corticosterone in plasma samples was calculated by using a standard curve run in duplicate on each plate. Plasma pools with two different corticosterone concentrations were included as internal controls on each plate. If the concentration was below the detection threshold, the determination was repeated with 10μl plasma. If the concentration was still below the detection threshold, the value of the lowest detectable concentration (1ngml-1) was assigned. Intraassay variation ranged from 4.5 to 10.8% and inter-assay variation from 9.6 to 17.6%, depending on the concentration of the internal control.
Tissue HSPs measurements
The HSPs measurements were conducted as described previously.31 Liver, heart and muscle tissues were utilized for total protein extraction. Briefly, 100mg frozen tissues were taken and protein was extracted according to the manufacturer’s instructions (Jiancheng Bioengineering Company, China). Extraction buffer was added to frozen liver tissue then homogenized properly using polytron. Afterward, it was centrifuged at 1200rpm for 10min at 4 °C. The supernatant that contains tissue extract was transferred into a fresh new Eppendorf tube for further extraction processes. Then, the lysate of tissue was diluted with diluent buffer and used for enzyme linked immune sorbent assay (ELISA). Similarly, the standards were prepared and ELISA was carried out as per instruction (Nanjing Jiancheng Bioengineering Company, Nanjing, China). The observance was measured at 450nm. The assay was carried out for HSP25, HSP72 and HSP90 protein concentration in tissues extracts.
Assessment of tissue oxidative stress
Lipid peroxidation in liver, heart and muscle were evaluated by measuring the amount of malondialdehyde (MDA) as described previously in our publication 32 using available commercial MDA kit. The MDA was measured in a UV spectrophotometry at 532nm as described in the manufacturer’s instructions. Approximately, 0.5g of tissues were homogenized in 4.5ml of ice-cold PBS buffer for preparation of testis homogenate, the homogenates were then centrifuged for 10min at 3000 rpm and the supernatant was kept at -20 ºC until analyzed. The levels of MDA in the tissue were expressed as nmol/g tissue. The glutathione peroxidase (GPx), Superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH) were also measured as described in our earlier publication.32 Briefly, about 1 g of testis tissues were cut into small pieces then homogenized in ice-cold normal saline (0.85%, pH =7.4) (1:9, wt/v) with an Ultra-Turrax (T8, IKA-labortechnik Staufen, Germany). Tissues homogenates were centrifuged at 1,000g for 15min at 4°C, and the supernatants were collected. The supernatants were used for the assays of SOD, GPx, CAT and GSH. SOD activity was measured and the specific activity was expressed in terms of units for milligrams of protein.
Real-time PCR and gene expression
About 100mg of liver and muscle were ground in liquid N2, and a portion of about 50 to 100mg were used for extraction of RNA using TRIzol total RNA kit (Invitrogen, Biotechnology Co, Ltd, Carlsbad, CA, USA) according to the manufacturer's instruction. Two approaches were taken to ensure that all the total RNA preparations are free of genomic DNA contamination. Firstly, total RNAs were treated with 10U DNase I (Rnase Free, D2215, Takara, Japan) for 30min at 37°C, and purified according to the manufacturer’s protocol. Secondly, the primers for the reference gene were designed to span an intron, so any genomic DNA contamination can be reported easily with an extra product in the melting curves for real-time PCR. For liver and muscle glucocorticoid metabolic genes expression, real-time PCR was performed in Mx3000P (Stratagene, USA) according to the previous publication.33 Mock RT and No Template Controls were included to monitor the possible contamination of genomic and environmental DNA at both RT and PCR steps. The pooled sample made by mixing equal quantity of RT products (cDNA) from all samples was used for optimizing the PCR condition and tailoring the standard curves for each target gene, and melting curves were performed to insure a single specific PCR product for each gene. The PCR products were sequenced to validate the identity of the amplicons. Primers specific for 11β-HSD1 and 11β-HSD2 (Table 3) were synthesized by Geneary (Shanghai, China). A MOUSE GAPDH was used as a reference gene for normalization purposes. The method of 2−ΔΔCt was used to analyze the real-time PCR data.34 The mRNA abundances were presented as the fold change relative to the average level of the control group.
Statistical analysis
Descriptive statistics analysis was performed to check the normality and homogeneity of variances before using parametric analyses. Body weight, food intake, organs weight, blood lipids profile, CORT, tissue HSPs, antioxidant biomarkers as well as the relative quantitative data of gene expression were analyzed by one-way ANOVA using SPSS 17.0 for Windows, followed by a least-significant difference (LSD) test for individual comparisons. A P-value ≤0.05 was considered significant.
Food intake, body weight, and organs weight
The treatment of GA significantly (P<0.05) decreased food intake compared to the control (Figure 1A). In addition, the treatment GA significantly (P<0.01) decreased body weight body weight compared to the control group (Figure 1B). However, the treatment of GA did not alter organs weigh (Figure 1C).
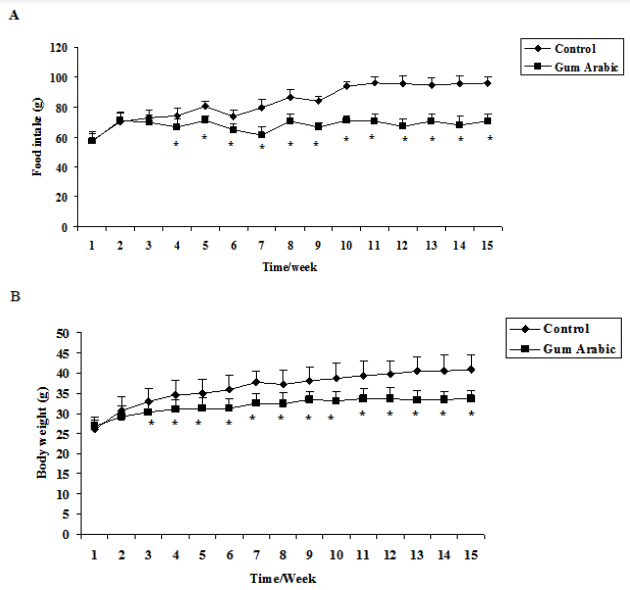
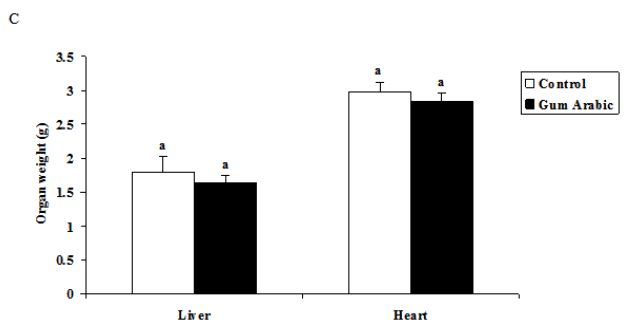
Figure 1 Effect of GA treatments on food intake (A) body weight (B), body weight; and organs weight (C). The values are the means ± SEM, n=10/group.
Plasma lipid profile and blood glucose
The treatment of GA significantly (P<0.05) decreased blood glucose concentrations when compared to the control group (Table 1). Similarly, the supplementation of GA significantly (P<0.05) reduced total cholesterol (P<0.05) and LDL compared to the control group whereas, increased plasma HDL good cholesterol concentrations compared to control (Table 1). No significant differences were observed both in plasma triglycerides and VLDL concentration regarding GA treatment.
|
Group |
Triglyceride (mg/dL) |
Total cholesterol (mg/dL) |
HDL (mg/dL) |
LDL (mg/dL) |
VLDL (mg/dL) |
Glucose (mmol/L) |
|
Control |
45.3±3.05a |
73.1±2.2a |
56.57±2.40a |
77.42±5.10a |
11.56±4.21a |
9.70±.43a |
|
Gum |
38.7±2.5a |
51.3±5.14 b |
68.42±3.15b |
34.81±2.16b |
9.52±0.13a |
5.04±0.60b |
Table 1
Tissue HSPs protein contents and plasma corticosterone concentrations
The treatment of GA significantly (P<0.01) increased liver HSP 25, HSP72 and HSP90 protein contents when compared to the control group (Figure 2A). Likewise, administration of GA significantly (P<0.01) increased heart HSP72 and HSP90 but not HSP 25 protein contents when compared to the control group (Figure 2B). In addition, the treatment of GA significantly increased muscle HSP72 and HSP90 protein contents when compared to the control group (Figure 2C). Moreover, the treatment of GA significantly (P<0.05) decreased plasma CORT concentrations compared to the control group (Figure 2D).
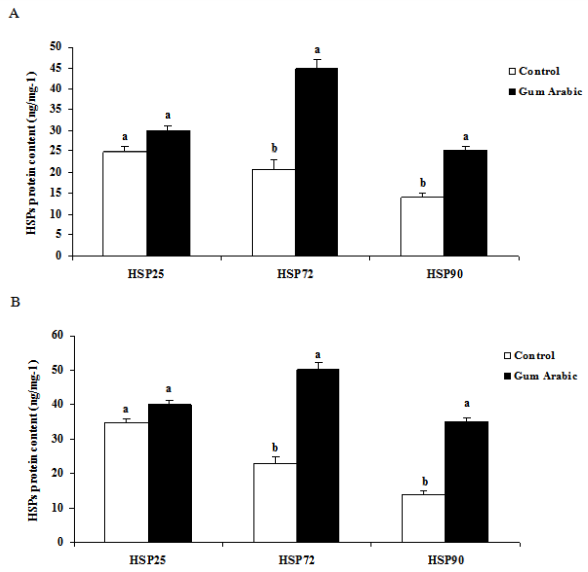
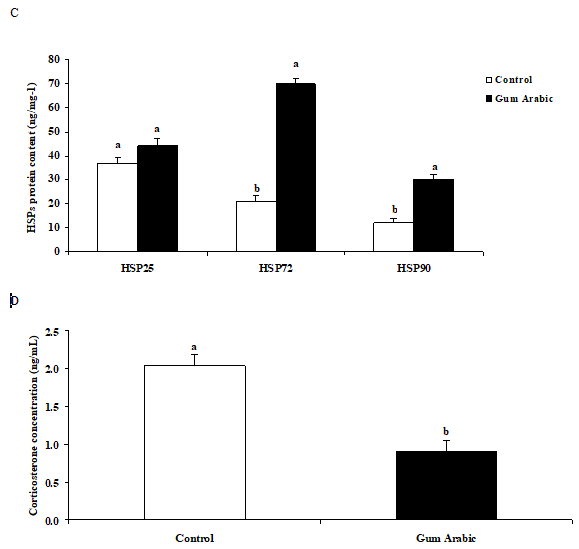
Figure 2 Effect of GA treatments on liver HSP25, HSP72 and HSP90 (A), muscle HSP25, HSP72 and HSP90 (B), heart HSP25, HSP72 and HSP90 (C) and plasma corticosterone concentrations (D). The values are the means ± SEM, n=10/group. Bars with different letters are significantly different at P<0.05.
Effect of GA treatment on hepatic oxidative stress
In this study, the treatment of GA significant (P < 0.05) reduced hepatic MDA levels compared to the control. However, the treatment of GA significantly (P<0.05) increased hepatic activities of CAT, GPx and SOD compared to the control group (Table 2).
|
Parameters |
Control |
Gum |
|
MDA (nmol/g wet tissue) |
27.52 ± 4.53a |
15. 51 ± 5.10b |
|
GSH (mg/g/g wet tissue) |
65.47 ± 5.24a |
101.4 ± 6.53b |
|
GST (μmol/g wet tissue) |
226.150 ± 31.02b |
268.3 ± 16.39b |
|
GPx (IU/g wet tissue) |
27.5 ± 3.05a |
51.3 ± 5.01b |
|
CAT (μmol H2O2 consumed/mg protein/min) |
594 ± 6.7a |
471.5 ± 5.23b |
|
SOD (u/mg protein) |
964.63 ± 6.14b |
1275.81 ± 7.65a |
Table 2
|
Target genes |
Gene bank number |
Product Size |
Primer sequences |
|
GAPDH |
NM_008084.2 |
141 |
F: 5′-ACATGGTCTACATGTTCCAGTA-3′ R: 5′-GGAGTCTACTGGTGTCTTCA-3′ |
|
11β-HSD1 |
NM_008288.2 |
302 |
F: 5′-AGCAACCAGAGATAGGCAGC-3′ R: 5′-ACACCTCGCTTTTGCGTAGA-3′ |
Table 3 Sequences of primers used for Q.PCR
Hepatic, heart and muscle 11β-HSD1 and 11β-HSD2 mRNA expression
In the present study, treatment of GA significantly (P<0.05) downregulated hepatic11β-HSD1 (Figure 3A) and muscle 11β-HSD1 (Fig. 3B) mRNA expression compared to control. On the other hand, the treatment of GA significantly (P<0.05) increased hepatic11β-HSD2 (Figure 3B) mRNA expression compared to control. However, no changes were observed in muscle 11β-HSD2 mRNA expression regarding GA treatment (Figure 3C).
No information was found in the previous studies regarding to the effect of Gum Arabic (GA) on the heat shock proteins (HSPs). Previous reports indicate that the HSPs are strongly induced via various types of stress35 whereas; they are expressed very low under normal conditions.36 GA is known to serve as a dietary fiber that has a potential health benefits.37 In the present study, we reported for the first time that the administration of GA to the normal mice significantly increased hepatic and cardiac HSP 25, HSP72 and HSP90. The increased tissue HSPs may be protect mice from several diseases including heart disease,38 vascular diseases39 such as atherosclerosis,40 and Muscular dystrophy.11 In addition, the elevation of HSPs in liver may protect the liver41 from stenosis,42 lipid accumulation 43 fatty liver disease.44 Interestingly, upregulation of HSPs were associated with increases in oxidative stress enzymes activities resulted in increases in CAT, SOD and GPx and decreases in MDA levels. The increase of HSPs could therefore, indicate the enhancement of antioxidant capacity10,45,46 which in turn protects the tissues from deleterious effects.47 These findings are consistent with our previous study which showed the antioxidant potentiality of GA and its role in tissue protection.48 In addition, the treatment of GA significantly increased muscle HSP72 and HSP90 protein contents. This findings support previous studies that increases of heat shock protein contents decreases oxidative stress,49 thus protects the tissues from cellular injury. 50
High circulating levels of low-density lipoproteins (LDL) and total cholesterol are considering the major risk factor for atherosclerosis.51 In the present study, the supplementation of GA increased tissue HSPs which were correlated with decreases in plasma total cholesterol, LDL-c concentrations whereas increased HDL-c. In humans, decreased serum HSP72 concentrations were associated with the occurrence of coronary artery disease.52 In experimental mouse model of atherosclerosis, the increased HSP72 concentrations were found to reduce lesion progression and enhanced features of plaque stability.53 The increased tissue HSP72 levels, could therefore, may represent a potential therapeutic target for hyperlipidemia related diseases.
Glucocorticoids (GCs) are known to play a vital role in the regulation of multiple biological processes, including stress responses,54 inflammatory responses, 55 immune response 56, energy balance 57 and development of obesity. 58 11 β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) is an intracellular gate-keepers of tissue GCs action. The treatment of GA downregulated hepatic and cardiac11β-HSD1 mRNA expression associated with decreased in plasma corticosterone concentrations. On the other hand, the treatment of GA increased hepatic11β-HSD2 mRNA expression. The suppression of 11β-HSD1 is fundamental in the treatment of metabolic syndrome59,60 and its related conditions including obesity61 atherosclerosis, 62 insulin resistance,63 type 2 diabetes millets 64 and nonalcoholic fatty liver disease.52 On the other hand, upregulation of 11β-HSD2 may contribute in resistant of metabolic syndrome.65 11β-HSD1 inhibitors are found to improve lipid profile,66 blood glucose,67 and improved adipose tissue functions.68 GA, therefore may pocesses the potential health benefits via inhbition of 11β-HSD1.
In conclusion, administration of GA increased tissue HSPs contents, increased antioxidant capacity and suppressed 11β-HSD1 mRNA expression associated with reduction of plasma CORT concentration. Thus indicate that GA may have a potentiality to protect tissue through enhancement of HSPs and antioxidant capacity. Further studies are required to clarify the mechanism of action through which the GA increases HSPs.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The study is a collaboration projects between University of Nyala, Nyala, Sudan, Medical and Cancer Research Institute, Nyala, Sudan; University “G. d’Annunzio” of Chieti-Pescara, Italy; Islamia University, Bahawalpur, Pakistan and National Ribat University, Khartoum, Sudan, Darfur University College, University of Khartoum and Medical and Cancer Research Institute.
Declare if any conflict of interest exists.
None.

©2021 Ahmed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.