International Journal of
eISSN: 2381-1803


Review Article Volume 14 Issue 6
1Department of Botany, School of Life Sciences, North-Eastern Hill University, India
2Department of Botany, School of Life Sciences, North-Eastern Hill University, India
Correspondence: Diana Kharkongor, Algal Ecology Laboratory, Centre for Advance studies in Botany, Department of Botany, School of Life Sciences, North-Eastern Hill University, Shillong- 793022, Meghalaya, India
Received: August 25, 2021 | Published: December 2, 2021
Citation: Kharkongor D, Ramanujam P. Effect of photon irradiance and temperature on carotenoids accumulation in four species of Trentepohlia (Trentepohliales, Chlorophyta). Int J Complement Alt Med. 2021;14(6):291-297. DOI: 10.15406/ijcam.2021.14.00575
Carotenoids the principal pigment of the Subaerial algal genus Trentepohlia was found to increase with increasing photon irradiance in culture. Maximum carotenoids accumulation was observed in Trentepohlia culture kept at 120??mol photons m-2sec-1. By lowering the temperature, carotenoids accumulation in Trentepohlia increased and progressively increased with decreasing temperature. Optimum production of carotenoids in all four Trentepohlia species was observed at 5°C. In all the four dominant species studied, it was observed that the amount of chlorophyll content was more than that of carotenoid content in culture. The ratio of carotenoid to chlorophyll content usually increased from the fourth week to the sixth week and then remained constant. Carotenoids and chlorophylls increased simultaneously along with culturing time and carotenoids were maximum at the end of culturing time. Comparing amongst the four species of Trentepohlia, T. diffracta contained the highest amount of carotenoids in culture followed by T. arborum and T. abietina, least amount of carotenoids was observed in T. umbrina.
Keywords: trentepohlia, subaerial algae, photon irradiance, temperature, carotenoids
Algae possess chlorophyll (a, b, c and d) and other types of accessory pigments such as carotenoids which aid in the capturing of light energy from the sun for photosynthesis. It is known that the content of chlorophyll a relating to the pigment level remain almost the same in all algal groups, but chlorophyll b and c changes along with the change in carotene level. It is reported that depending on the algal species and environmental conditions, variations occurred in the level of different pigments especially an increase in carotenoids level under stress conditions. It has been suggested that carotenoids function as a passive light protecting filter and as an accessory pigment in transferring energy and oxygen.1–8 In plants and algae, carotenoids were synthesized in the chloroplasts around the nucleus and their accumulation was effected only when they were exposed to some stress factors.9 Abe et al.12 has demonstrated the accumulation of carotenoids under high light conditions, for protecting the thylakoid membrane against photo oxidative damage by absorbing an excess amount of solar energy. In photosynthetic organisms, these carotenoids played important roles in either absorbing energy or protecting cells from photo-oxidative damage.10 Subaerial microalgae unlike the other algae are constantly living under stress due to exposure to higher light intensity during day time and unavailability of water. Hence, formation of high carotenoids is one of the strategies to survive in harsh environmental conditions.
Trentepohlia Martius 1817, a common genus of subaerial green algae grow well in areas with adequate light intensity.11 Growth as well as carotenoid accumulation in Trentepohlia aurea and Trentepohlia arborum had been studied by Abe et al.12 and Chen et al.13 respectively. Abe et al.12 studied the effect of light, temperature and organic nutrient on T. aurea, where they reported enhanced growth and carotenogenesis by switching the illumination from 3000 to 10,000 lux. Many workers tried to increase the carotenoid content of Trentepohlia in culture by manipulating different stress conditions. Ong et al.14 reported it from desiccation stress, while Tan et al.15 and Abe et al.12 achieved higher carotenoids accumulation in nutrient starved condition and others reported it by manipulating light intensities.15,16
The present work aimed at evaluating the influence of different factors like photon irradiance and temperature on the growth and carotenoids accumulation in four dominant species of Trentepohlia under laboratory conditions.
Algal samples
Four dominant species of Trentepohlia were collected from four different substrata in Shillong Meghalaya; viz. Trentepohlia diffracta (Krempelhuber) Hariot from cemented wall, Trentepohlia arborum (C. Agardh) Hariot from rock surface, Trentepohlia umbrina (Kutzing) Bornet from iron electric pole (since it was difficult to segregate T. umbrina from other groups of algae growing along with it on bark of trees, hence it was collected from an electric pole where the growth of other groups were negligible) and Trentepohlia abietina (Flotow) Hansgirg was collected from bark of Eucalyptus tree. Identification of collected Trentepohlia species was done using standard monographs and literature.17–22
Medium composition and preparation
Modified Bold Basal Medium23 was used for culturing the four dominant species of Trentepohlia (Table 1).
|
NH4Cl |
1000mg |
|
KH2PO4 |
175mg |
|
K2HPO4 |
75mg |
|
MgSO4.7H2O |
25mg |
|
NaCl |
25mg |
|
EDTA |
50mg |
|
KOH |
30mg |
|
FeSO4.7H2O |
5mg |
|
H3BO3 |
11mg |
Table 1 Composition of the modified BBM
Inoculation of algal samples
Equal colony of the alga from pure culture was inoculated in the conical flasks (triplicates) containing modified BBM medium. These were then kept in the culture rack at 20, 40, 80 and 120??mol photons m-2 sec-1 provided by cool fluorescence light and photoperiod of 16:8 light and dark periods to examine the effect of photon irradiance on growth of each species of Trentepohlia. Similarly in order to study the effect of temperature on four species of Trentepohlia the conical flasks (Triplicates) were kept in an incubator at different temperature (5°C, 15°C, 25°C, 30°C and 35°C) at 40??mol photon m-2s-1 and photoperiod of 16:8 light and dark periods.
Pigment analyses
10mg of the dried harvested culture of each species of Trentepohlia was taken and the pigments were extracted in 80 % acetone and absorbance was measured using a Cary 100 UV-visible spectrophotometer. Chlorophylls and carotenoids were calculated using the formula given by Wellburn.24
Chlorophyll a (ca) (mg/g) = 12.21 A663-2.81 A646
Chlorophyll b (cb) (mg/g) = 20.13 A646-5.03 A663
Total carotenoids (Cx+c) (mg/g) = (1000 A470-3.27 ca-104cb)/198
Car/Chl =Total carotenoids/ (Chlorophyll a + Chlorophyll b).
Effect of photon irradiance on carotenoids production in four species of Trentepohlia
Total carotenoid content: Carotenoids the principal pigments of subaerial algae were found to increase with increasing photon irradiance in all four species studied. Maximum carotenoids accumulation was observed in Trentepohlia culture kept at 12??mol photons m-2 sec-1 and lowest content of carotenoids was observed in all four species of Trentepohlia kept at 20??mol photons m-2 sec-1. Comparing in between the species of Trentepohlia, carotenoid content was maximum in T. diffracta (20.45mg/g) at 120??mol photons m-2sec-1 in the sixth week old culture, followed by that in T. arborum (18.98mg/g) and T. abietina (18.77mg/g) and least amount was recorded in T. umbrina (16.20mg/g) (Figure 1).
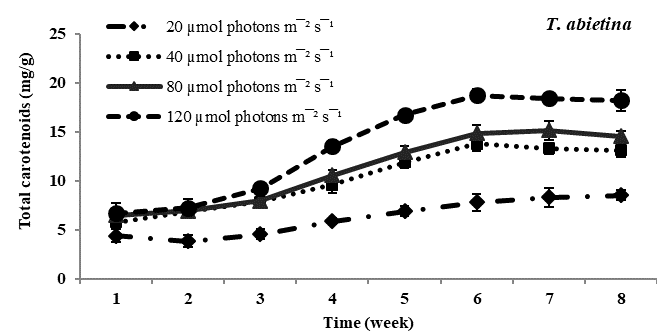
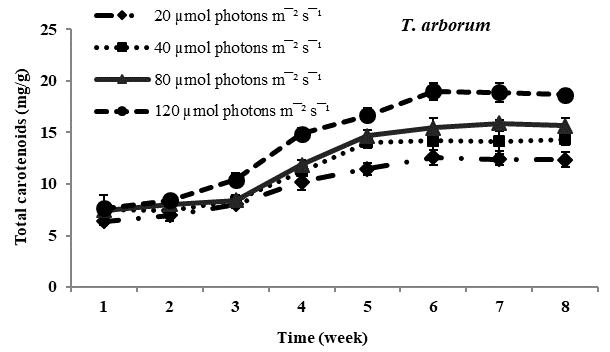
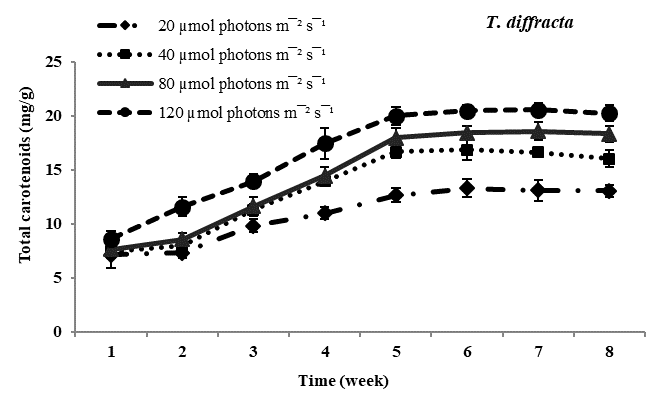
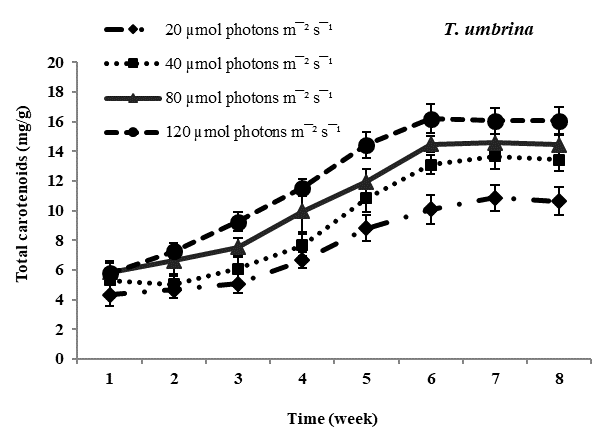
Figure 1 Total carotenoid content (mg/g) in Trentepohlia abietina, T. arborum, T. diffracta and T. umbrina at different.
Carotenoids/chlorophylls ratio: In all the four dominant species studied, it was observed that the amount of chlorophyll content was more than that of carotenoid content in culture. The ratio of carotenoid to chlorophyll content usually increased from the fourth week to the sixth week and then remained constant. Carotenoids and chlorophylls increased simultaneously along with culturing time and at high photon irradiance of 120??mol photons m-2sec-1 carotenoids accumulation was maximum. At lower photon irradiance the ratio almost remained constant throughout the culturing period.
Effect of temperature on carotenoids production in four species of Trentepohlia
Total carotenoid content: Carotenoid content in all four species of Trentepohlia was maximum at lowest temperature of 5°C and was minimum at 35°C. In all the experimental temperature, carotenoids increased gradually from third week till sixth week of incubation and then gradually declined from the seventh week in all the four species studied. Comparing amongst the four species, maximum accumulation of carotenoids at low temperature of 5°C was observed in T. diffracta, followed by that in T. arborum, T. abietina and minimum was in T. umbrina. There was no significant difference in amount of carotenoid content in the four Trentepohlia species grown at 15°C, 25°C and 35°C (Figure 2).
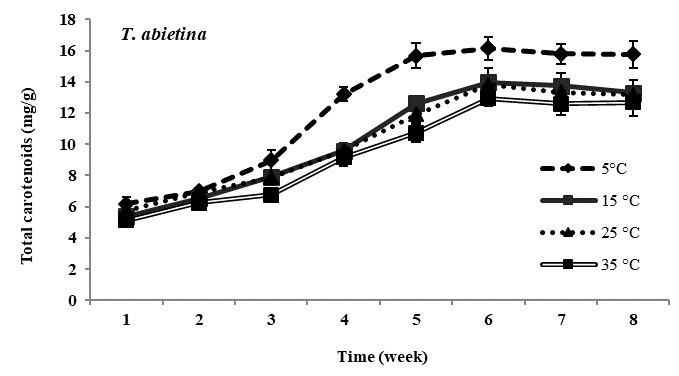
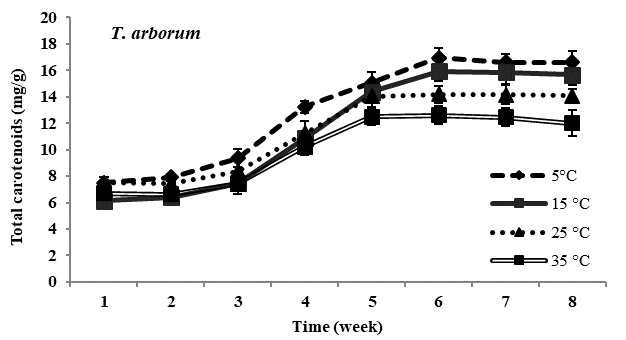
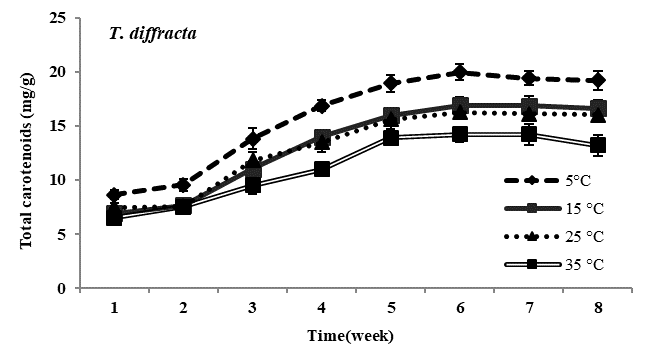
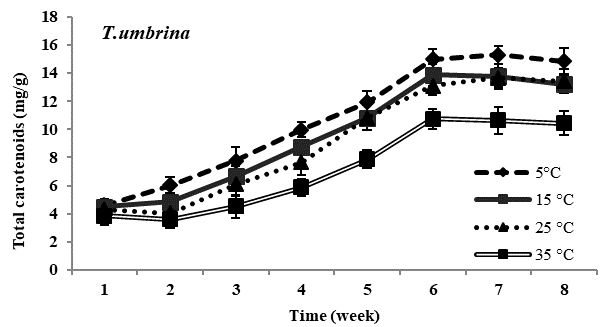
Figure 2 Total carotenoid content (mg/g) in Trentepohlia abietina, T. arborum, T. diffracta and T. umbrina at different temperature.
Carotenoids/chlorophylls ratio: As in the case of Trentepohlia grown at different photon irradiance, the ratio of carotenoid to chlorophyll content in the four species generally increased from the fourth week to the sixth week and then became constant or started declining gradually. Carotenoid by chlorophyll content increased along with culturing time and the ratio value increased when Trentepohlia was grown at 5°C and it was observed to be maximum in T. diffracta.
Effect of different photon irradiance and temperature could be observed on growth of four species of Trentepohlia. Optimum growth rate in this study was observed on culture kept at 40??mol photons m-2 sec-1 irradiance and 25°C temperature. With increase in photon irradiance and temperature growth of Trentepohlia decreased significantly, similarly low growth rate was observed at lower temperature and lower light intensity. This signifies that 40??mol photons m-2 sec-1 and 25°C are the optimum irradiance and optimum temperature for better growth of the four species of Trentepohlia in culture. The significant decrease in growth rate at highest photon irradiance could be due to photoinhibition which resulted in decreased rate of photosynthesis and consequently led to decline in biomass production and on the other hand lower light intensity led to lower light requirements essential for growth of Trentepohlia. In microalgae, low irradiance could limit photosynthesis,25 but high irradiance caused photoinhibition.26 In addition to light, temperature also affected the photosynthetic activity and the growth of microalgae.5 Optimum temperature of 25°C had been reported for many microalgae and some species of Trentepohlia as well. Abe et al.12 reported that the optimum irradiance of T. aurea was at 43??mol photons m-2 sec-1 and optimum temperature to be 25°C. They also demonstrated significant decrease in growth rate by decreasing and increasing the temperature below and beyond 25°C in T. aurea. Similar trend could be observed the present case as well. According to many authors, the negative effect of suboptimal growth temperature on algal metabolism and efficiency got strengthened when low temperatures were accompanied by high irradiances.27–30 Similarly growth of all four species of Trentepohlia was inhibited at suboptimal temperature as well as at high temperature in this study. Magana and Villareal31 reported that the optimum temperature for growth in a dinoflagellate Karenia brevis as 15°C -30°C, the growth rate increased when the irradiance was increased from 31 to 67??mol photons m-2 sec-1, and the growth rate reduced significantly at temperature below 15°C. Several workers demonstrated that biochemical processes are temperature-sensitive and at temperatures deviating far from the optimum, the absorbed light energy could not be converted into carbohydrates efficiently. Moreover at low temperatures the turnover of enzymes was reduced, and thus the metabolic activity of the algae became lower which led to a lower growth rate and a reduced need of substrate.5,32,33
The carotenoid content of the four species of Trentepohlia varied with both the light regime and temperature. Carotenoid content in all Trentepohlia species increased when cultures were exposed to high irradiance of 120??mol photons m-2 sec-1 compared to those kept at lower light intensity. The increased production of total carotenoids at higher irradiance agrees with the findings by Abe et al.12 where carotenogenesis in T. aurea was enhanced by switching the light intensity from 43 to 143??mol photons m-2 sec-1. It is well reported that microalgae has a tendency to accumulate carotenoids at higher light stress as a protective measure to prevent the cells from photo damage.34–37 Hence a higher carotenoid/chlorophyll ratio was observed at high irradiance in all the four species of Trentepohlia in this study. Several unfavourable environmental conditions such as nutrient deficiency, intense irradiation, and excessive photosynthesis lowered the rate of electron transfer and in turn caused photo-oxidative damage.38 Active oxygen molecules, generated by excess photooxidation caused by high light irradiance, do apparently triggered synthesis of carotenoids as part of a cellular strategy aimed at cell protection against oxidative damage.39
With decreasing temperature all the four species of Trentepohlia increased in carotenoid content whereas a reduction in carotenoids was observed at higher temperatures. The maximum production of carotenoid content at 5°C clearly indicated that low temperature greatly enhanced the accumulation of carotenoids in the four species of Trentepohlia where a maximum carotenoid/chlorophyll ratio was also observed. Effect of low temperature on carotenoids production in microalgae had been demonstrated by some workers.6,40,41 It was reported that at low temperature the algae are more susceptible to photooxidation.6,42 Hence, high amount of carotenoids are accumulated in some microalgae as a protective measure to overcome photoinhibition and oxidative stress.43-45 Aksu and Tugba46 reported denaturation of the enzymes responsible for carotenoids biosynthesis at high temperature. Therefore, this could also be the reason responsible for the decline in production of carotenoids at higher temperature in the four species of Trentepohlia.
Comparing amongst the four species, highest carotenoids accumulation in culture was recorded in T. diffracta. According to Hu et al.47 the synthesis of lipids in microalgae varied with the species used. Amongst the species of Dunalliela, D. Salina is known to produce the highest content of carotenoids compared to other species. Gomez and Gonzalez48 demonstrated the existence of a genetic basis supporting the physiological differences amongst the strains. They further demonstrated that the strain was the main factor controlling total carotenoids accumulation and carotenoids/chlorophylls ratio in seven strains of Dunaliella salina cultivated under laboratory conditions.49 Li et al.50 successfully identified 99 unigenes encoding key enzymes involved in carotenogenesis in T. jolithus, they also demonstrated and explained why this aerial microalga could survive and spread in cold, high altitude habitats. They also provided new insights into the regulation of photosynthesis and suggested potential strategies for optimizing conditions favouring growth and carotenogenesis. In the present work also, the variation in growth rate and in amount of carotenoids accumulation exhibited by different species could thus be attributed to the genes encoding enzymes responsible for carotenoid biosynthesis in different species of Trentepohlia.
Production of maximum carotenoids was seen at the 6th week or 7th week culture which was the ending stage of log phase and early stationary phase respectively. This indicated that culture stages also greatly governed the phase of maximum production of carotenoids. Gao et al. (51), in their study with a microalga Vischeria stellata, demonstrated the gradual increase in content of β-carotene with prolongation of culture time, resulting in its exclusive production at the end of cultivation. The reason for maximum accumulation of carotenoids in matured culture could also be due to depletion of nitrogen as biomass increased at the end of culture time. Several authors documented the accumulation of carotenoids in aeroterrestrial algae under nitrogen-limiting conditions (52, 23 & 14). Hence we can conclude that by altering the temperature and photon irradiance Trentepohlia a genus of subaerial microalgae can be used for mass production of carotenoids for various neutraceutical and pharmaceutical purposes.
We are thankful to North Eastern Hill University for the financial support and to the Head of Botany Department, for providing us all the laboratory facilities.
This work had been carried out in the Algal Ecology Laboratory in North-Eastern Hill University, a Central University of India.
Therefore, “we the authors hereby declare that there is no conflict of interests regarding the publication of this article”.
“We also declare that this manuscript has not been published elsewhere and is not currently under consideration by another journal. The submission of the Manuscript is approved by the Institution.
Dr. Diana Kharkongor and Dr. Papiya Ramanujam.
None.

©2021 Kharkongor, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.