International Journal of
eISSN: 2381-1803


Research Article Volume 15 Issue 1
1 Department of Geriatrics Medicine, Sociedad de Acupuntura Médica de España, Spain
2 Pediatrician, Chief of Integrative Pediatric Oncology Unit Hospital Sant Joan de Déu, Spain
Correspondence: Beltrán Carrillo Manrique, Geriatrics Medicine, President of SAME (Sociedad de Acupuntura Médica de España)
Received: January 20, 2022 | Published: February 21, 2022
Citation: Manrique BC, García EM. COVID-19 and Acupuncture: is there a new line of therapeutic research? Int J Complement Alt Med. 2022;15(1):51-63. DOI: 10.15406/ijcam.2022.15.00590
The pandemic produced by COVID-19 has surprised almost all health and socio-economic systems worldwide with its high degree of infectivity and mortality. In short time we have learned many aspects of the epidemiology, pathophysiology and clinic of the virus responsible, SARS-Cov-2, and the disease it induces. COVID-19 is known to produce in some patients the development of an excessive immune response and hyper inflammation, damaging various organs and sometimes causing death. There is no effective treatment available at the moment, other than life support, and for this reason different revealing therapeutic options are being tested but pending confirmation of their effectiveness and safety in well-designed clinical trials. We describe the anti-inflammatory and immunomodulatory effects of Acupuncture, as well as its preclinical and clinical effectiveness in different conditions that have similar COVID-19 immunological disorders. The proven biological plausibility of Acupuncture, together with its acceptable level of clinical effectiveness and safety, make this medical technical procedure an option to try in this health emergency situation. We propose an adjusted classification of the clinical presentation of COVID-19 and an appropriate adjuvant Acupuncture protocol for each phase of the disease.
Keywords: acupuncture, COVID-19, SARS-CoV-2, immunomodulation, hyper inflammation, sepsis, treatment protocol
Coronaviruses (CoV) are viruses that can cause pathology in mammals, birds, and humans. They are large RNA viruses, with the largest genome among known RNA viruses and they owe their name to their spherical shape from which spicule in the shape of a crown protrude. Human coronaviruses were first isolates in the 1960s. Seven types of human coronaviruses are known, four of them (HCoV-229E, HCoV- OC43, HCoV-NL63, HCoV-HKU1) are the causative agent of common cold along with rhinoviruses. Three other new coronaviruses have emerged in the 21st century, having caused serious outbreaks with acute respiratory distress syndrome (ARDS): SARS-CoV (China, 2002), MERS-COV (Middle East, 2012)1 and the current SARS-CoV-2.2
IN December 2019 in Wuhan, Hubei Province (China) an outbreak of pneumonia cases caused by a new b-coronavirus, SARS-CoV-2, was first detected.3 From China it quickly disseminates around the world, putting health, economic and political systems to the limit. A pandemic that is generating a situation of unknown global crisis and has more than 375,000 confirmed cases in 196 countries and more than 16,000 deaths.4 On February 11, 2020, the world Health Organization (WHO) gave the official name of the infection. The name was coronavirus disease 2019, and it was abbreviated COVID-19. In the abbreviated name, “CO” stands for “corona”, “VI” for “virus” and “D” for “disease”. Previously, the way to refer to this disease was “new coronavirus 2019” or “2019-nCoV”.5
The natural reservoir of all coronaviruses are bats and through an intermediate mammal they can infect humans. Transmission between persons was observed from the first reported cases of COVID-196 taking measures of early confinement and exhaustive general hygiene to limit contagiousness within the population. Other possible risk factors are being studied such as age, s ex, underlying disease, viral inoculation dose, genetic susceptibility,7 environmental factors such as smoking.8
SARS-CoV-2 data confirm that it is a highly infectious virus with a high transmission capacity. It is transmitted primary through Flügge droplets from the respiratory secretions of infected persons when they exhale. In addition, when these Flügge droplets fall, they deposit on surfaces, where other people can contract the infection if they touch those objects or surfaces with their hands and take them to eyes, nose of mouth.9
This high infectivity rate is enhanced by its high incubation time (between 1 and 14 days), which provides it with a great pre-symptomatic transmissibility. After the disease is cured transmission is also possible. There are also cases of fecal-oral transmission due to the digestive symptoms that may happen during the disease. No cases of perinatal or breast milk transmission have been reported to date.10
Pathophysiology of COVID-19
The SARS-CoV-2 infection induces an immune response in two phases,11 the first phase the viral pathogenicity predominates and, the second, in which the pathology is mainly due to the excessive immune response of the host. Being the transition from one phase to another, both from a clinical and analytical point of view, progressive. During incubation and in the non-severe phase of the disease, a specific adaptive immune response is required to try to eliminate the virus and limit progression to more advanced stages of infection. The second phase begins when the protective immune response is altered.12 As a sign of the dysfunction of the immune system we can observe the decrease in CD3+ and CD+ T cells. The dysregulation of the immune system will trigger a series of disproportionate and negative immune responses for different affected tissues. Starting through the complex signaling recruitment adapters that develop a molecular cascade that activates nuclear transcription factor kb (NF-kb) and the production of type I interferons (IFN-a/b) and a series of pro-inflammatory cytokines ( IL-1b, IL-6, macrophage colony stimulating factor (MCSF), IP-10, MCP-1, hepatocyte growth factor (HGF), interferon-g (IFN-g), tumor necrosis factor-a (TNF-a). The cytokine profile associates with severe COVID-19 resembles that seen in secondary hemophagocytic lymphohistiocytosis (sHLH): increased IL-2, IL-7, granulocyte colony-stimulation factor, interferon-g, inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-a and TNF-a.13 Not much is known about sHLH a hyperinflammatory syndrome characterized by fulminant hypercytokinemia and fatal multiorgan failure, which can occur in 3.7 – 4.3% of cases of viral sepsis in adults. sHLH presents with sustained fever, cytopenias and hyperferritinemia, and lung involvement in 50% of the patients (SDRA).14
According to a multicenter retrospective study of 150 cases of COVID-19 in Wuhan, it suggests that mortality could be due to viral hyperinflammation and increases ferritin and IL-6 would act as markers of severity.15 Other predictors of severe SARS-CoV-2 disease are elevated D-dimer on admission, lymphopenia, and increase high-sensitivity troponin 1.16
The current treatment of COVID-19 is supportive, and respiratory failure due to ARDS is the leading cause of mortality. Accumulated evidence suggests that a subgroup of patients with severe COVID-19 may have a cytokine release syndrome (CRS).17 Early identification and treatment of hyperinflammation, using all existing therapies with acceptable safety profiles is important to address the immediate need to reduce mortality.18
Clinical classification
SARS-Cov-2 infection does not progress similarly in everyone who comes in contact with it. The interest in the clinical classification lies in being able propose the most appropriate therapeutic approach for each patient according to the phase in which they are. According to the classification proposed by Shi et al.,19 there is an initial asymptomatic incubation period (PHASE I),20 similar to the incubation periods of SARS and MERS,21 although there is evidence that this period can have a highly variable period depending on the inoculation dose.22 Of the infected and confirmed cases, between 60-80% have a mild to moderate symptomatology (PHASE II), characterized by fever, dry cough, asthenia and myalgias, which is adequately managed on an outpatient basis with symptomatic treatment. The remaining 20-40% have a serious clinical course with multiple organ involvement (PHASE III), predominantly at the respiratory level, needing between 5-8% critical care for respiratory failure and sepsis. With a mortality rate of 3.6% (95% CI: 3.5 to 3.7) although these mortality rates are based on the number of confirmed cases of infection, which does not represent the current mortality rate.23 The WHO has recently reported that the time between the onset of symptoms and death varies from 2 to 8 weeks.24
The classification proposed by Siddiqi & Mehra25 also distinguishes 3 periods. Period I (mild or early infection) ranging from inoculation to the establishment of the disease characterized by nonspecific symptoms such as malaise, fever, dry cough, headache, anosmia or ageusia. At the analytical level, the blood count can reveal lymphopenia and neutrophilia without other significant abnormalities. Period II (moderate or with respiratory involvement without hypoxemia (IIA) or with hypoxemia (IIb)) with establishment of respiratory disease with worsening of symptoms and appearance of radiological signs of viral pneumonia (infiltrates or opacities in characteristically bilateral ground glass) and analytical abnormalities (exacerbation of lymphocytopenia, moderate elevation of D-dimer and transaminitis), as well as the onset of elevation of systematic markers of inflammation, but not in a extreme way, highlighting a normal to low procalcitonin. At this stage, most patients with COVID-19 would need to be hospitalized for observation and treatment. A relevant clinical aspect in this period is the appearance of hypoxemia as a marker of the progression of the infection and the possible need for mechanical ventilation resulting from the pulmonary inflammation hyperresponse that, if not interrupted, will lead to Period III (severe or systematic hyperinflammation). Clinically, in this stage, multiorgan involvement is observed in many patients with a progressive evolution towards worsening and hemodynamic instability and sepsis. This period is characterized by a significant elevation of systemic inflammation markers (IL-2, IL-6, IL-7 granulocyte colony stimulating factor, macrophage inflammatory protein 1a, TNF-a, C-reactive protein (PCR), ferritin and D-dimer). Ferritin is the key indicator of macrophage activation. D-dimer rises similarly to antiphospholipid syndrome along with thrombocytopenia and coagulation disturbances.
Current therapeutic approach
To date, there is no effective antiviral treatment against SARS-CoV-2. Antiviral drugs and systemic corticosteroids useful in other viral infections have not been shown to be valid for COVID-19. Recent studies have revealed attractive therapeutic options, although they have yet to be confirmed in studies in rigorous preclinical and clinical models.26 Some authors highlight the use of corticosteroids at the beginning of the hyperinflammatory response and not in the early stages, where their early use could provoke viral replication.27 Remdesivir, a RNA antiviral, has been used to successfully treat the first case of COVID-19 in the USA.28 Even though it is the most promising antiviral currently being investigated, its efficacy and safety are not yet established. The viral load in COVID-19 has been shown to decrease significantly, in one case, after administration of Lopinavir/Ritonavir,29 although the efficacy of this drug mixture is nor definitively established. Several studies have found that Hydroxychloroquine can inhibit some steps in the replication of various viruses, with a potent effect on the infection and spread of other viruses of the coronavirus family.30 Hydrochloroquine also has immunomodulatory effects, suppressing the production and release of TNF-a and IL-6.31 Currently, the efficacy, dose and safety of Hydroxychloroquine for the treatment and prevention of COVID-19 are not established, and more data is needed on whether its in-vitro activity against SARS-CoV-2 corresponds to clinical efficacy. Recent publications show that the combined use of Hydroxychloroquine and Azithromycin are more effective in eliminating the virus.32
In the line of suppressing different proinflammatory cytokines, various quasi-experimental protocols are been initiated at hospital level to modulate the excessive immune response and thus avoid the damage produced by the CRS, this phenomenon been responsible for the fatal outcome in some cases of infection by SARS-CoV-2.
Proposal of clinical classification for the approach with Acupuncture
Inspired by Siddiqi & Mehra, we propose a 5-stage classification (Figure 1) to try to identify the different clinical characteristics and enhance the most appropriate therapeutic approach for each phase. Acupuncture can be potentially useful in all phases as a complementary and synergic treatment to try to improve the patient’s global condition, prevent progression and help during convalescence.
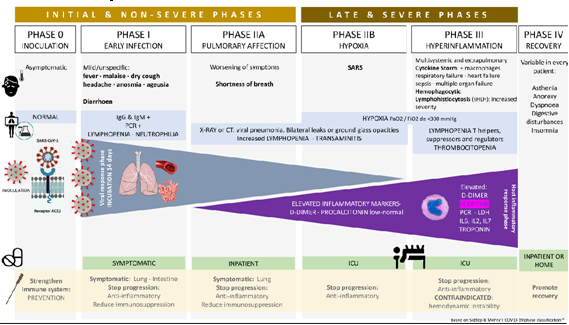
Figure 1 Adaptation Siddiqi & Mehra.25
IIa: analytical, clinical and radiological alterations without hypoxemia.
IIb: clinical worsening, severe analytical and radiological changes, appearance of hypoxemia.
Phase III: period characterized by an excessive immune response (cytokine storm) with multi- system involvement in different degrees, including septic shock.
Phase IV: recovery. As of today, the long-term evolution of people affected by COVID-19 is still an enigma. Given the predominantly pulmonary involvement of the acute phase, we can suspect that most of the sequelae will be at this level. Radiological studies of patients in remission phase show gradual reabsorption of acute phase lesions on computed tomography, but cord-like high density shadows indicative of fibrosis.33 Based on epidemiological, immunological and clinical evidence, it is expected that patients who survive SARS-CoV-2 infection may suffer, as the most serious complication, pulmonary fibrosis.34 There is preclinical evidence that moxibustion has a positive effect on pulmonary fibrosis comparable to prednisone, attributed to the inhibition of transforming growth factor-b (TGF-b) and IFN-g, measured both at mRNA and protein level.35
Therapeutic approach with acupuncture
The therapeutic approach at the present time is being developed as the pathophysiology, and clinical characteristics of COVID-19 are been discovered. It seems reasonable to guide investigation to the acute phase (non-severe and severe phases) and to the recovery phase. In these phases it is proposed that the synergistic technical medical procedure of Acupuncture can add a beneficial effect to conventional treatment:
The initial / non-severe phases, where viral pathogenicity predominates (phases 0, I and IIa). Looking for measures that improve the host’s immune response in order to limit the progress of the disease to more severe phases.
The late/severe phases, where the immune response and hyperinflammation (cytokine storm) to limit multi-organ damage.36
The recovery phase, the sequelae of the inflammation will favour the appearance and progression of pulmonary fibrosis that can condition the lung capacity and quality of life. The objective is to limit the extension and progression of fibrosis and promote recovery of lung function.
Why acupuncture may be of interest as an adjunctive treatment in COVID-19
Acupuncture is a medical therapeutic procedure widely used both in the east and western countries for different conditions, mainly in pain, but also in other non-pain conditions. The mechanisms of action of Acupuncture have been extensively studied, and it has been seen that it has a modulating effect at different levels. One of the most recognized is the immunomodulatory and anti-inflammatory effect.37,38,39,40 There are recent reviews on how Acupuncture attenuates the immune response to various conditions, such a surgical stress on cognitive function,41 postsurgical ileus,42 inflammatory bowel disease,43 allergic rhinitis,44 depression,45 anxiety,46 migraine,47 spinal cord injury,48 knee surgery in the elderly,49 sepsis,50 lung injury secondary to limb reperfusion ischemia,51 arthritis,52 rheumatoid arthritis,53 cerebrovascular disease,54,55 cognitive decline,56 myocardial ischemia57 and obesity.58,59
Basis for the application of acupuncture in the initial phase of COVID-19: boosting host immunity Acupuncture has been extensively studied to understand its mechanisms of action. Far from traditional concepts, Acupuncture is now known to allow the restoration of homeostasis by the regulation it exerts between the autonomic nervous system, innate immunity and other systems.60 The autonomic nervous system is considered one of the most important mediators of acupuncture stimulation, since it can interconnect peripheral somatosensory stimulus with responses of different organs through networks of the central nervous system. The vagus nerve, which regulates the function of different organs, has been the most studied target of the possible effects of Acupuncture.61 Vagus nerve stimulation by Acupuncture is known to have three major pathways with strong anti-inflammatory effects: the hypothalamic-pituitary- adrenal axis, the cholinergic anti-inflammatory pathway, and the sympathetic-splenic-anti-inflammatory pathway.62
Reviewing the literature, we can find many articles that show the action of Acupuncture and the modulation of the immune system. The abbreviation of each Acupuncture point is broken down in the final annex:
We can summarize the action of the most frequently studied acupoints in the modulation of the immune system to justify their use in the initial phase or COVID-19 (Table 1).
|
Acupuncture and Immunity |
ST36 |
LI11 |
CV6 |
CV4 |
BL23 |
BL60 |
KI27 |
LU7 |
LI4 |
BL12 BL13 GV14 |
Other: ST9, HT1, LU5, GB1, GB20, BL15, BL17 |
Study Model |
|
↑ IL-2 |
|
|
|
|
|
|
|
|
|
|
|
Human69 |
|
↑IFN-γ |
|
|
|
|
|
|
|
|
|
|
|
Animal64 |
|
↑ NK |
|
|
|
|
|
|
|
|
|
|
|
Human69 |
|
↑ T cell CD3+ |
|
|
|
|
|
|
|
|
|
|
|
Human63,70 |
|
↑ T cell CD4+ |
|
|
|
|
|
|
|
|
|
|
|
Human63,66,70 |
|
↑ CD4+/CD8+ |
|
|
|
|
|
|
|
|
|
|
|
Human66,70 |
|
↑ IgG, IgM, and IgA |
|
|
|
|
|
|
|
|
|
|
|
Human70 |
Table 1 Acupuncture and immunity
Bases for the application of acupuncture in the late phases of COVID-19: anti-inflammatory action to control the “cytokine storm”
Sepsis is a clinical syndrome characterized by a generalized inflammatory response similar to what occurs in COVID-19. The etiology is multiple, although the course of all patients and the systemic pathophysiological changes are similar among them. In this sense, we find multiple studies that evaluate the effects of Acupuncture in animal models of sepsis. These studies induce sepsis by injection of lipopolysaccharide (LPS) or D-galactosamine, or by ligation and posterior cecum perforation (CLP). A systematic review of these pooled studies on the effect of Acupuncture on ST36 has recently been published (Lai, F et al71). They find and analyse 54 studies publishes between 2006 and 2018 (31% in the last 5years). Most of these studies use EA, although also Manual Acupuncture (MA), at different points: ST36, PC6, BL13 and LI4. The results show that Acupuncture has a beneficial effect in the evolution of sepsis and manages to reduce the degree of injury on different target organs, such as lung,72,73 kidney74,75 brain76,77,78 digestive tract,79,80 liver,81 cardiovascular system82 and with positive data o survival.83
These effects are justified by modulating the immune response, reducing proinflammatory cytokine levels (TNF-a, IL-6, IL-1b),84 increased anti-inflammatory cytokines (IL-10),85 decreased oxidation,86,87 microcirculation improvement.88
The most likely mechanisms to achieve these effects are through the cholinergic anti-inflammatory pathway,89,90 with its effect on macrophages, inhibiting the release of pro-inflammatory cytokines, without ruling out other routes such as dopaminergic,91,92 Nrf2/ACE,93 ERK1/2, heme-oxygenase-1 (HO-1) pathway,94,95 stimulation of the mitogen-activated protein kinase p38 (p38MAPK),96,97 inhibition of the expression of group of high mobility proteins B1 (HMGB1) that promotes the expression of ghrelin.98
Many of these results are observed in studies that initiate Acupuncture before the onset of septic symptoms. Some authors consider that once the excessive immune response in initiated, it may be more difficult to control the immunological alterations and clinical presentation. Having therefore relevance when considering the application of Acupuncture in the initial phase of the disease to limit the progression of COVID-19 to severe stages.
Some of the studies in animal models published in the medical literature are:
Human studies have also shown the effectiveness of Acupuncture in the management of sepsis:
We can summarize the action of the most studied acupoints in sepsis that would justify their use in late stages of COVID-19 in Table 2.
|
Acupuncture and Sepsis |
PC6 |
ST36 |
LI11 |
LI4 |
BL13 |
GV20 |
ST25 ST37 |
GB34 |
CV4 |
Jiaji |
Auricular |
Study model |
|
↓ plasmatic nitrous oxide |
|
|
|
|
|
|
|
|
|
|
|
Animal100, 107, |
|
↓ TNF-α |
|
|
|
|
|
|
|
|
|
|
|
Animal100, 105, 107, 108, 110,111,112, 114, 117 |
|
↓ NF-kβ |
|
|
|
|
|
|
|
|
|
|
|
Animal105,117 |
|
↓ IL-6 |
|
|
|
|
|
|
|
|
|
|
|
Animal105,107,108, 110,111,112,117 |
|
↓ IL-1β |
|
|
|
|
|
|
|
|
|
|
|
Animal107,108, |
|
↓IFN-γ |
|
|
|
|
|
|
|
|
|
|
|
Animal111,112 |
|
↑ IL-10 |
|
|
|
|
|
|
|
|
|
|
|
Animal107 |
|
HMGB1 inhibition |
|
|
|
|
|
|
|
|
|
|
|
Animal109,115 |
|
Antioxidant effect |
|
|
|
|
|
|
|
|
|
|
|
Animal114,117 |
|
↑ CD3+ T cell |
|
|
|
|
|
|
|
|
|
|
|
Animal116 Human119,120 |
|
↑ CD4+ T cell |
|
|
|
|
|
|
|
|
|
|
|
Animal116 |
|
↑ CD4+/CD8+ |
|
|
|
|
|
|
|
|
|
|
|
Animal116 Human119,120 |
|
↑ IgG, IgM and IgA |
|
|
|
|
|
|
|
|
|
|
|
Human119 |
|
Blood pressure |
|
|
|
|
|
|
|
|
|
|
|
Animal100, |
|
Decrease Acute Lung Injury |
|
|
|
|
|
|
|
|
|
|
|
Animal72, 73, 102, 105, 113 |
|
Nephroprotection |
|
|
|
|
|
|
|
|
|
|
|
Animal74, 75, 102, 107, |
|
Hepatoprotection |
|
|
|
|
|
|
|
|
|
|
|
Animal81,104, 106 |
|
Brain protection |
|
|
|
|
|
|
|
|
|
|
|
Animal76, 77, 78, 110, 117 |
|
Intestinal edema improvement |
|
|
|
|
|
|
|
|
|
|
|
Animal79, 80, 103, 115, 116 |
|
Survival Increase |
|
|
|
|
|
|
|
|
|
|
|
Animal83, 99, 108, |
|
Cholinergic pathway |
|
|
|
|
|
|
|
|
|
|
|
Animal99, 106 |
|
↓ proinflammatory cytokines in CNS |
|
|
|
|
|
|
|
|
|
|
|
Animal110 |
|
Dopaminergic pathway |
|
|
|
|
|
|
|
|
|
|
|
Animal91, 92, 109, 110 |
|
↓ lactulose / mannitol excretion |
|
|
|
|
|
|
|
|
|
|
|
Human118 |
|
↓refeeding time |
|
|
|
|
|
|
|
|
|
|
|
Human118 |
|
↓ICU stay |
|
|
|
|
|
|
|
|
|
|
|
Human119 |
Table 2 Acupuncture and sepsis
a: pinna Shell points that stimulate the vagus nerve and the cholinergic anti-inflammatory pathway
Bases for the application of acupuncture in the recovery phase: effect on the formation of fibrosis: There are different studies at different tissue levels that show how Acupuncture prevents the formation of fibrotic tissue:
IN a spontaneous hypertension model in rats comparing EA with losartan, it is observed that EA at BL17 and/or BL23 manages to control blood pressure, expression levels of tissue inhibitory metalloproteinases (TIMP-1), plasminogen activated inhibitor-1 (PAI-1) and muscle actinlysa-a (a-SMA), as well as the histopathological changes observed at kidney level after staining with hematoxylin/eosin in a similar way as losartan.123
The therapeutic effect of EA on bruised skeletal muscle has been confirmed in various clinical studies. In this publication, it is observed how EA at ST36 helps the regeneration of bruised skeletal muscle, relieving fibrosis and increasing the size of the regenerated myofibrils. EA increased the number of M2 macrophages and decreased those of M1, as well as decreases the levels of IFN-g and increases levels of IFN-a, IL-4, IL-13 that contribute to the regeneration of injured tissue and the less formation of fibrosis.124
The effects of Acupuncture on renal interstitial fibrosis were studied in an animal model of chronic renal failure. The results show that, Acupuncture stimulation on BL23, BL20 and GV4, reduces levels of TNF-a, ILK, TGF-b, IL-8, IL-1b, Smad and iNOS expression, acting on the TGF-b/Smad pathway.125
In an animal model of muscle injury, it is observed how EA at BL40 reduces the degree of muscle fibrosis and promotes tissue regeneration in the initial phase, this effect being associated with the regulation of the expression of collagen I proteins and metalloproteins matrix 2.126
The anti-inflammatory effects of EA at ST36 allows, in addition to reducing the local post-surgical inflammatory response (decreases TNF-a), attenuate angiogenesis (decreases vascular endothelial growth factor (VEGF)) and alleviates the formation of adhesions in part by cholinergic anti-inflammatory mechanisms.127
Creating a cervical intervertebral disc lesion in rats, it was seen that EA at BV14 inhibits cellular apoptosis of fibrous ring by suppression of TNF-a, TNF receptor 1 and caspase-8 positive cells and the expression of integrin b1 and Akt mRNA.128
In an animal model of muscular injury, EA at ST36 and trigger points, achieves significantly higher levels of serum activity of total SOD and total antioxidant capacity, and lower levels of MDA. In the EA group, the diameter of the myofibrils was uniform with a regular arrangement. EA significantly decreased fibrosis formation. The mechanisms are attributed to the improvement of blood flow and antioxidant capacity.129
These studies show how Acupuncture reduces fibrosis formation in different organs that are undergoing a period of high inflammation and oxidation. Modulation of the immune system has been considered the key step in the therapeutic effect of Acupuncture and Moxibustion.130 EA can inhibit the induction and transmission of pain signals and consequently, through its antinociceptive and anti-inflammatory effects, rebalance neuro-immune-endocrine interactions.131
The studies previously analysed show the beneficial effect of Acupuncture on the immune system. In the health of non-serious disease situations, it boosts immunity, while in chronic and acute disease, when there is a situation of hyperinflammation, it manages to ”appease” this excessive and negative immune response in different organs and for the whole individual. We therefore have studies on the biological plausibility of Acupuncture in inflammation.
Proposed protocol of intervention with acupuncture for COVID-19
The studies analysed show the beneficial effect of Acupuncture on the immune system. In light of the data, it would be interesting to assess and include Acupuncture as a complementary treatment for the management of patients with COVID-19 to try to prevent or contain the poor evolution related to an uncontrolled immune response and a state of hyperinflammation. These are the fields in which most preclinical and clinical research has been carried out in the last 20 years in the field of Acupuncture.
There is not yet any study that analyses the effect of Acupuncture in patients with COVID-19, nor do they exist for almost all treatments that are been empirically used at the moment. They are underway, according to clinical evidence and from the urgency that these pandemic warrants. From China, favourable data on the use of traditional Chines medicine, mainly phytotherapy, has been published, although data on the use of Acupuncture is still lacking, some recommended protocols for different stages of the disease have transcended,132 that more or less coincide with the acupoints used in the studies analysed above.
Following the guidelines proposed by the paper of Sun y Zhou,133 the experience of the Chinese health authorities and, taking into account the published medical literature, we propose the following Acupuncture protocol as a synergistic and complementary treatment in COIVD-19. We would like to include, in Phase 0, a simple protocol for the prevention or strengthening of the immune system that could also be applied to health care professionals who are in the front line of serving COVID- 19 patients with a high risk of becoming infected. We propose daily puncture with needle insertion time of 30 minutes.
Phase 0: period from inoculation to the development of first symptoms of disease. Everyone with potential, recognized and non-symptomatic epidemiological contact could be included. In this phase we could include uninfected health professionals, to strengthen their immune response globally.
Suggested acupoints: acupoints can be needled, leave continuous stimulation with press tack needles and/or use moxibustion. EA can also be used at low frequency (2-3Hz) if there is no medical contraindication:
Phase I: period of early infection, asymptomatic or with mild symptoms: fever, cough, headache, myalgia and arthralgia.
Suggested acupoints: acupoints can be needled, continuous stimulation with press tacks, moxibustion is not recommended:
Prevalence of respiratory symptoms: cough and / or odynophagia.
Prevalence of digestive symptoms: abdominal pain, bloating, nauseas, vomiting, or diarrhoea.
Phase II: period with lung involvement (viral pneumonia), with clinical and radiological worsening.
Suggested acupoints: acupoints can be needled, continuous stimulation with presstack, moxibustion is not recommended.
IIa (without hypoxemia) add: TE5 + GB41
IIb (hypoxemia) add: LU10, LI11 y ST44
Phase III: period characterized by an excessive immune response, hyperinflammation (cytokine storm), with multi-system involvement including septic shock. Only recommended stimulation with needle. Do not use moxibustion. Acupuncture is contraindicated in hemodynamic instability.
Suggested acupoints: in this phase, some patients may be placed in a prone position to promote ventilation. The appropriate acupoints should be chosen depending on the position of the patient.
Phase IV: recovery.
Suggested acupoints: aimed at promoting the recovery of the patient during convalescence ST36 and BL13.
ST36 and BL13
KI3, BL17, BL20 and BL23
If the patient presents: nausea, bloating and loose stools.
CV4 and CV6: moxibustion can apply
If the patient presents: insomnia, night sweats, flushing, dry mouth, restlessness, dizziness, muscle weakness or oliguria.
SP6, KI10, LI3, LI8 y LU6
In all phases of active infection, it is recommended to add bilateral auriculotherapy by puncturing, with continuous stimulation (press tack needles), sedation and analgesic points such as shenmen, and points in the shell area related to vagus nerve stimulation. In the area of the cymba shell, the kidney and small intestine points, and in the area of the shell cavum, the lung and heart points.
Continuous stimulation points (press tack needles) should always be changed no later than 72 hours after puncturing under strict aseptic conditions, like all Acupuncture needles.
Acupuncture in children with COVID-19
In children, the infection is usually milder, but in some cases, it can be complicated and require hospital admission or even intensive care. In children under 7years of age, it is recommended to use press tack needles to stimulate the proposed acupoints described above, leaving them inserted up to 72hours, and following replacement. To improve tolerance to treatment, points can be placed unilaterally to try to reduce the number of points and increase tolerance to Acupuncture. IN collaborative children over 7 years old, it can be considered to use acupuncture needles and / or press tack needles.
The data and therapeutic protocols of COVID-19 are constantly evolving. The severity of the disease requires rapid interventions. It is a highly transmissible pandemic with a considerable mortality rate in some age groups, and its fatal evolution is related to an excessive immune response and a state of hyperinflammation. Acupuncture has known mechanisms of action extensively studies in the field of immunomodulation and anti-inflammatory effect in various clinical presentations, including sepsis.
The COVID-19 health emergency has put our health systems, our economy and our lifestyle in check. It has forced us to react urgently, applying empiricism of known drugs (such as hydroxychloroquine and azithromycin), of off-label drug trials or other non-patentable substances, such as vitamin C or B3. We are doomed to put aside strict clinical trials to try to save lives, with all the knowledge we have, within acceptable margin of safety and efficacy and a low risk/benefit ratio, in this scenario of uncertainty.
Acupuncture is an old acquaintance, non-patentable, cheap medical procedure, very safe in expert hands and that in light of the reviewed publications, can offer a potential benefit that deserves to be evaluated in patients, designing a single center or multicenter study that combines efforts, from all possible medical approaches, to deal with COVID-19.
None.
The authors are professionally engaged in Medical Acupuncture.
None.

©2022 Manrique, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.