International Journal of
eISSN: 2381-1803


Research Article Volume 7 Issue 1
1Departamento Ciências Naturais, Universidade Estadual do Sudoeste da Bahia, Brazil
2Departamento Química e Exatas, Universidade Estadual do Sudoeste da Bahia, Brazil
3Departamento Ciências Biológicas, Universidade Estadual de Santa Cruz, Brazil
Correspondence: Cristina Pungartnik, Departamento Ciências Biológicas, Universidade Estadual de Santa Cruz, Laboratório de Biologia dos Fungos, Rodovia Ilhéus Itabuna, Km-16, Salobrinho, Ilhéus, Bahia, Brazil, Tel +557336805438
Received: February 20, 2017 | Published: May 17, 2017
Citation: Marisco G, Santos RX, Aguiar R, Brendel M, Pungartnik C (2017) Antifungal Potential of Terpenes from Spondias Purpurea L. Leaf Extract against Moniliophthora Perniciosa that causes Witches Broom Disease of Theobroma Cacao. Int J Complement Alt Med 7(1): 00215. DOI: 10.15406/ijcam.2017.07.00215
The present in vitro study evaluates antifungal properties of Spondias purpurea plant extract against Moniliophthora perniciosa. Fractions of Ethanol Crude Extracts (CE) from S. purpurea were tested for their antifungal activity. CE inhibited mycelial growth of M. perniciosa by 60%at a dose of 10mg/mL, while survival of broken hyphae of the fungus was inactivated by 90% at a dose of 8mg/mL. Fractionation of CE yielded a terpene containing sub fraction (FA) with the highest fungicidal activity. FA acted as oxidant and its application induced oxidative stress in M. perniciosa that may be the responsible for the observed induced cell death. The chromatographic profile obtained with FA of S. purpurea revealed a variety of terpenes, which included Spathulenol (14.2%), Linolenic acid (8.4%), trans Caryophyllene (6.9%), and Alpha-muurolene (6.9%).This study describes for the first time effects of terpenes of S. purpurea related to induced oxidative stress in fungus. S. purpurea, therefore, may be a natural source for isolation of antifungal compounds against M. perniciosa. This characteristic may be useful in application as botanical fungicide in biological control.
Keywords:moniliophthora perniciosa, spondias purpurea, biofungicide, terpenes, natural products, theobroma cacao
Moniliophthora perniciosa (Stahel) is a hemibiotrophic phytopathogenic fungus which infects cacao (Theobroma cacao L.) and causes witches broom disease.1 It has introduced serious socio-economic and environmental problems 2 by reducing cacao production in Southern Bahia, the main cacao producing region in Brazil.3 There have been numerous efforts in the last 25years, to halt the spread or to eradicate this phytopathogenic fungus. Several strategies in minimizing M. perniciosa induced crop damage have emerged:
However, growth of T. cacao seed lings subjected to increasing levels of copper (Cu) is negatively affected17 and Cu also is known to cause environmental problems.18 Synthetic pesticides and fungicides may have various side effects on human health, including carcinogenicity, teratogenicity and residual toxicity.19 Therefore, biological control appears to be beneficial in reducing effects of witches’ broom disease to economically viable levels and in decreasing the use of chemical fungicides that may cause collateral damage.6 The chemical components occurring naturally in variety of plants in Brazilian ecosystems are a potential source of bioactive materials.20 Plant metabolites and plant-based pesticides and fungicides increasingly appear to be a promising alternative to synthetic pesticides as their application promises minimal environmental impact.21,22 Therefore, they are helpful for the development of effective products for pest control.23 including pathogenic fungi24‒26 Spondias purpurea L. (Family: Anacardiaceae), originating from the region of Mexico and Central America,27 is widely present in north eastern Brazil,28 Popularly known as Seriguela or Ciruela, it is traditionally used in the treatment of anemia, diarrhea, dysentery and skin infections.29 In Brazil it is also used against diabetes and high cholesterol.30,31 Its economic importance is also derived from the production of fruits, juices, jams, ice cream and alcoholic beverages.32
In vitro studies showed antifungal activity of S. purpurea as aqueous leaf extract decreased the germination of Rhizopus stolonifer conidia.33 Aqueous extracts of leaf and stem could inhibit spore formation of Colletotrichum gloeosporioides,34 while methanol leaf extract and powders inhibited the mycelial growth of Fusarium oxysporum while hexane extract decreased its sporulation.25 Based on increasing public concern about pesticide residues in food products, as well as about soil-degrading effects of fungicides, the availability of a sustainable, environmental-friendly method for disease control in T. cacao is highly desirable35 and generally, biocontrol appears to be one of the few viable approaches.36 Therefore, we aimed to evaluate in vitro the antifungal potential of S. purpurea extract against the plant pathogen M. perniciosa.
Plant extract
The plant S. purpurea was collected from Vitória da Conquista, Bahia (UESB, 140 52' 49" S 400 47' 34"W). Voucher specimen were identified and deposited in the herbarium at UESB (number 6457). The leaves were macerated with a pistil after drying in circulating warm air at 40°C for 2days. A 100 g powdered sample was exhaustively extracted with 99.9% ethanol. The ethanol was removed using a rotary evaporator to produce a dry powder of the Crude Extract (CE) after filtering with filter papers.
Fractionation of the crude extract of S. purpurea
Extracts with different polarities were obtained by liquid-liquid partition from the Crude Ethanol Extract of S. purpurea (CE).37 Initially 1.0 g of CE was dissolved in MeOH: H2O (4:1), then it was homogenized for 5min and filtered. The resulting filtrate was acidified with H2SO4 and then partitioned with CHCl3. The organic phase generated the chloroform fraction (FA), while the acidic alcoholic hydro phase was basified and partitioned with CHCl3: MeOH (3:1).The organic phase, after removing the solvent, generated the basic chloroform fraction (FB), while the hydro alcoholic phase contained the basic hydro-alcoholic fraction (FC). The filtration residue was extracted with EtOAc. After solvent evaporation the fraction of EtOAc (FD) was obtained.
In vitro antifungal assay
M. perniciosa strain and growth conditions: Strain ALF553 of M. perniciosa (deposited at Universidade Estadual de Feira de Santana, at Feira de Santana, Bahia, Brazil, access number CCBM000257)was used in all the experiments. Starter cultures of M. perniciosa were kept for 14 d at 25°C on solid agar media (CPD: 2% glucose, 2% peptone; 2% agar used as solidifying agent). Dikaryotic phase of growth was microscopically confirmed by the presence of clamp connections while species-specificity of M. perniciosa cultures was confirmed by PCR amplification of the actin gene (data not shown).38
M. perniciosa sensitivity to CE:The starter culture of M. perniciosa containing disc (2-3mm) was then transferred to CE-supplemented CPD (1, 10 and 20mg/mL CE). CE was diluted in 99.9% p.a. ethanol and the maximum ethanol concentration per agar plate was 5%. Plates were incubated at 25°C for 14days. The halo of M. perniciosa mycelial growth was photographed and measured. Photos are representative for a minimum of 3 experiments. Results are expressed as percent (N/Nox 100) of reduction of treated mycelium diameter (N) as compared to control (No). Data represents media and standard deviation of minimum 4 measurements per plate, (i.c. 95%) for at least3 plates. Ethanol-only control plates did not differ from control plates (CPD medium).
M. perniciosa survival curve to CE and sub fractions:Sensitivity of M. perniciosa broken hyphae to CE and its four sub fractions (FA, FB, FC, and FD) in either liquid or solid CPD was determined by the methodology described by Filho et al.,39 Briefly, two to three discs (2-3mm) were cut out from the periphery of the mycelium of the starter culture and transferred into 5mL of liquid CPD containing 1g of sterile glass beads (Sigma, G1277). After vigorous vortexing for 60s, 1 to 3mL of the resulting suspension was transferred to10mL fresh liquid media and incubated for 7d without agitation. From this liquid culture 1-3mL of M. perniciosa suspension was again vortexed and re-suspended in 10mL of liquid CPD. This suspension was used for M. perniciosa survival experiments in two different ways:
Phytochemical screening
The extracts (CE, FA, FB, FC, FD) of S. purpurea were subjected to qualitative chemical screening for the identification of the classes of alkaloids, flavonoids, tannins, saponins, terpenes and proteins, according to methodology described.41,42
Fluorescence assay
M. perniciosa hyphae were treated with 1mg/mL FA at 25°C for 1h. The hyphae were then washed twice with 0.9% saline. A one mL sample of the treated hyphae was then stained by addition of 1μL stock solution of dihydroethidium (DHE; Sigma-Aldrich) (1mg/mL) and mixed by inverting it. After incubation at 37°C for 30 min hyphae were washed thrice with 0.9% saline and re-suspended in 100μL of saline. One mL samples of non-treated and treated hyphae (with 4mM H2O2) were processed under the same conditions and were checked for oxidative/reductive stress. Images were captured using a 40X objective under bright field as well as under fluorescent filters using IM50 software (Leica). The photographs are representative for hyphae from at least three independent experiments.
Chromatographic analysis
The chemical constituents of fraction FA were analyzed using a Shimadzu QP5050A GC-MS, fitted with Shimadzu AOC-20i auto sampler. The GC-MS with (5%-phenyl) methylpolysiloxane DB-5 capillary column (30m x 0.25mm) with thickness of 0.25µm; carrier gas helium at 1.2mL/min with split less mode was used. Temperatures of the injector and detector were set at 280°C and 280°C respectively. The column temperature was programmed at 60°C (hold 1min) to 180 °C (hold 1 min) at 4°C/min and then at 260°C (hold 25min) at 10°C/min. The mass spectra were produced by electron impact at 70 eV. The constituents were identified by comparing retention indices (RI) with the corresponding reference data in Adams43 and by using the library of mass spectra of NIST11. C13 to C20 n-paraffin hydrocarbons mixture was used to obtain the RI. Also, injections of sample and standards in GC-FID, under the same conditions of analysis, were used to obtain the percentage of area.
Effect of S. purpurea CE on mycelial growth of M. perniciosa
The results of antifungal action of the crude extract (CE) against M. perniciosa are shown in Figures 1A & 1B. Radial growth of mycelia was inhibited by about 60% at 10mg/mL and total growth inhibition by CE was achieved at 20mg/mL.
Survival of M. perniciosa hyphae treated by CE and its sub fractions
When M. perniciosa hyphae were subjected to the crude extract at a dose of 8mg/mL they showed a high sensitivity, i.e., only 10% survival of colony forming units (Figure 1C). The survival of M. perniciosa hyphae after treatment with the fractions (FA, FB, FC, FD) of CE is shown in Figure 2. FA had highest antifungal activity at 1mg/mL followed by FD at 5mg/mL and further followed by FB. FC was not active at any concentration.
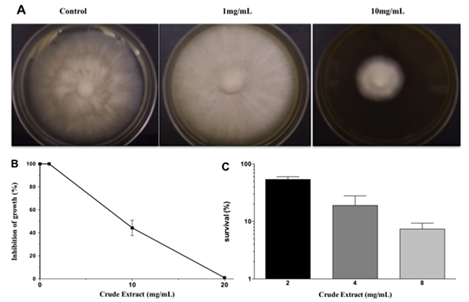
Figure 1 Effect on radial growth of M. perniciousa of different CE concentrations.
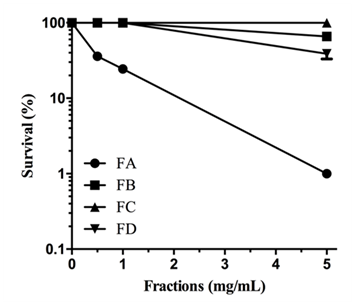
Figure 2 Sensitivity of M. perniciosa hyphae treated with S. purpurea CE (0.25, 0.5, 1 and 5mg/mL respectively) in liquid CPD media for 1 h and plated on CPD medium; number of colonies were counted after 7 d incubation at 25°C.
Phytochemical screening
S. purpurea CE and its three sub fractions exhibiting antifungal activity were phytochemically analyzed in a qualitative screening of the main secondary metabolites. Tannins were present in CE, FA and FB, while saponins were absentin FD. Alkaloids were present in CE and FB. However, terpenes could only be detected in CE and FA.
FA from S. purpurea induced oxidative stress in M. perniciosa
Vegetative growth on CPD (control mycelium without FA) (Figure 3A) was compared to mycelium growth after H2O2 treatment (4mM, Figure 3B) and FA (1mg/mL, Figure 3C) on the basis of observations obtained by fluorescent microscopy. Some oxidation was observed in non-treated mycelia (Figure 3A). Oxidation produced by reactive oxygen species (ROS) after H2O2 treatment (Figure 3B) was clearly higher in comparison to control. Oxidation induced by FA (Figure 3C) was much stronger when compared to positive H2O2 treatment control (Figure 3B).
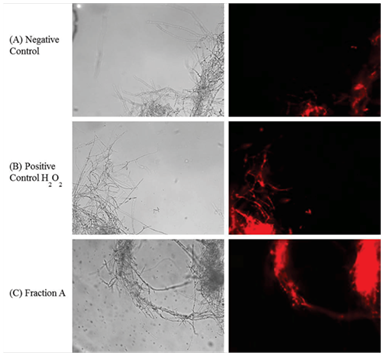
Figure 3 Visualization of oxidative stress induced by sub fraction A of S. purpureal leaf extract via fluorescence microscopy of DHE-stained M. perniciosa.
Chromatographic analysis
It lists the chemical constituents of FA, the sub fraction with the highest antifungal activity, identified by GC-MS and GC-FID. The chromatographic analysis of the study identified 14 components (78% of the sample), amongst them the presence of sesquiterpenes (56.09%) as major constituents; spathulenol (14.2%), trans-caryophyllene (6.9%) and alpha-muurolene (6.9%), in addition to free fatty acid, linolenic acid (8.4%).
Previous studies with aqueous extracts of S. purpurea leaves demonstrated the fungicidal potential on Rhizopus stolonifera33 and the aqueous extracts from leaf and stem totally inhibited the sporulation in Colletotrichum gloeosporioides.34 These results suggested further studies, e.g., to isolate and identify the compounds with fungicidal potential from the extracts. In this present study, S. purpurea CE totally inhibited the growth of the basidiomycete fungus M. perniciosa at 20mg/mL, and about 60% at 10mg/mL when applied to solid agar media. In the first attempt of isolation of compounds responsible for M. perniciosa cytotoxicity, CE was fractionated using polarity separation methodology.37 Three of 4 fractions FA, FB, and FC of CE had antifungal activity as they were cytotoxic against M. perniciosa (Figure 2). Fraction FD displayed no antifungal activity. The differential distribution of bioactivity among the fractions might be explained by the proportions of different bioactive components of each fraction, acting either in a synergetic or in potentiating way.44 Since FA showed the strongest inhibition of mycelial growth, we chose it for further analytical studies.
Phytochemical screening of CE and fractions can be correlated with cytotoxicity to M. perniciosa (Figure 2). While all secondary tested metabolites could be detected in CE and, per definition rendered 100% of cytotoxicity, only sub fraction FA that contains only terpenes was able to induce the same cytotoxicity. Phytochemicals contained in FB and FC could also induce cytotoxicity, however to a lesser degree than to one shown for CE and FA. The effect of plant extracts on fungal pathogens may be attributed to their content of secondary metabolites (e.g., alkaloids, phenolics, flavonoids and terpenoids) with known antifungal activity.45,46Antifungal activity of S. purpurea extract was associated with the concentration of sesquiterpenesas it inhibited the growth of mycelium of Fusarium oxysporum.25 Therefore, we only submitted FA to GC-MS analysis. Major constituents of FA were sesquiterpenes (56.09%), which could be associated with a high degree of cytotoxicity (Figure 2). Terpenes are known to induce membrane disruption of lipophilic compounds24 and are also known to induce ROS.47 Hence, a fluorescence assay with DHE was performed (Figure 3). Interaction of cytosolic DHE when oxidized by ROS (singlet oxygen, hydroxyl radicals, superoxide, peroxides and hydro peroxides) produces ethidium, which intercalates with DNA and emits bright red fluorescence at 605nm.48 Induction of oxidative stress after FA and H2O2 detected by DHE was observed (Figure 3). The amount of FA used in this study showed considerable higher oxidant activity, which effects mycelial growth of M. perniciosa in vitro (Figure 3C) in comparison to oxidative stress induced by H2O2 (Figure 3B). DHE-exposed M. perniciosa hyphae had shown a similar profile when subjected to a free radical-generating chemical treatment. Marisco et al.,49 had shown similar results in the eukaryotic model in S. cerevisiae (yeast) using H2O2 as pro oxidant. There is evidence for the role of endogenous ROS as a causative agent in mutagenesis and as a significant contributor in the mutational burden in microbes during periods of oxidative stress in which this response may be related to Cytotoxicity.50 Thus, endogenous ROS produced by oxidative metabolism of M. perniciosa can explain the basal oxidant activity observed in non-treated mycelia (Figure 3A). In short, our results show that crude ethanol extract of S. purpurea leaves is capable of inducing cytotoxicity in M. perniciosa (Figure 1). The fraction named FA was responsible for 100% mycelia inhibition in M. perniciosa (Figure 2), and chemical analysis revealed sesquiterpenes as its major component. The presence of terpenes could be correlated with oxidative stress produced by S. purpurea FA (Figure 3), which in turn, could be responsible for the cytotoxicity in the phytopathogen M. perniciosa (Figure 1 & 2). To our knowledge, this is the first description of S. purpurea terpenes, the major component found in leaf extracts, to be responsible for inducing oxidative stress and the correlation with cytotoxicity on M. perniciosa. As there still little effective products against witches' broom disease in T. cacao, and chemical fungicides like copper hydroxide pose problems in soil deterioration,16 the continuing search for antifungal bio products is warranted. Leaf extracts of S. purpurea had significant antifungal properties, hence this plant will be a good candidate for intensified in vitro and in vivo studies of its antifungal activities. Since S. purpurea is widely distributed in northeastern region of Brazil and the production of leaf extract with ethanol is quite simple, this might be a good base to develop a commercially viable product for the treatment of witches’ broom disease of cacao.
None.
Author declares there are no conflicts of interest.
None.

©2017 Marisco, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.